Abstract
Background
We aimed to investigate the therapeutic efficacy and safety of Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in platinum-resistant recurrence of ovarian cancer and peritoneal carcinomatosis, while our secondary endpoint was to establish any changes in quality of life estimated via the EORTC QLQ-30 and QLQ-OV28 questionnaires.
Methods
In this monocentric, single-arm, phase II trial, women were prospectively recruited and every 28–42 days underwent courses of PIPAC with doxorubicin 2.1 mg/m2 followed by cisplatin 10.5 mg/m2 via sequential laparoscopy.
Results
Overall, 98 PIPAC procedures were performed on 43 women from January 2016 to January 2020; three procedures were aborted due to extensive intra-abdominal adhesions. The clinical benefit rate (CBR) was reached in 82% of women. Three cycles of PIPAC were completed in 18 women (45%), and 13 (32.5%) and 9 (22.5%) patients were subjected to one and two cycles, respectively. During two PIPAC procedures, patients experienced an intraoperative intestinal perforation. There were no treatment-related deaths. Nineteen patients showed no response according to the Peritoneal Regression Grading Score (PRGS) and 8 patients showed minor response according to the PRGS. Median time from ovarian cancer relapse to disease progression was 12 months (95% confidence interval [CI] 6.483–17.517), while the median overall survival was 27 months (95% CI 20.337–33.663). The EORTC QLQ-28 and EORTC QLQ-30 scores did not worsen during therapy.
Conclusions
PIPAC seems a feasible approach for the treatment of this subset of patients, without any impact on their quality of life. Since this study had a small sample size and a single-center design, future research is mandatory, such as its application in addition to systemic chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Peritoneal carcinomatosis is a common condition in epithelial ovarian cancer and affects about 75% of women at the time of first diagnosis. Despite extensive frontline surgery and standard adjuvant chemotherapy with platinum-based treatments, around 80% of women diagnosed at an advanced stage will experience disease recurrence.1
Women showing recurrent ovarian cancer more than 6 months after first-line chemotherapy are considered to have platinum-sensitive disease. In this context, as shown in the final analysis of the randomized controlled study DESKTOP III, highly selected patients with isolated relapse of disease can benefit from cytoreductive surgery with respect of chemotherapy alone.2,3,4,5,6,7 Furthermore, the recent introduction of the minimally invasive approaches may further reduce the surgical morbidities in this subset of patients.8,9
On the other hand, recurrent ovarian cancer patients showing relapse within the first 6 months after completion of platinum-based chemotherapy are defined as platinum-resistant. According to the most recent guidelines, women experiencing platinum-resistant relapse, regardless of the extension and pattern of disease presentation, are offered salvage systemic chemotherapy as the only recommended therapeutic option, given the short survival.10 Even if some retrospective studies have demonstrated that secondary cytoreductive surgery (SCS) prolongs post-relapse survival compared with chemotherapy alone in patients with isolated platinum-resistant recurrent ovarian cancer,11,12 palliative intravenous chemotherapy remains the only option in this specific subgroup of women.
However, the efficacy of intravenous chemotherapy against peritoneal carcinomatosis could be affected by several factors, including poor blood supply within solid nodules13 and elevated intratumoral fluid pressure.14 In this context, some initial promising experiences have been published regarding the role of surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in treating peritoneal relapse within 6 months from completion of primary platinum-based chemotherapy by means of improving the locoregional control of disease.15,16,17,18 However, many of these studies are focused on resectable disease, leaving systemic chemotherapy as the only option in cases of peritoneal carcinomatosis.
Over the last decade, a new technique has been offered to patients affected by platinum-resistant recurrence of ovarian cancer and peritoneal disease, which allowed increased bioavailability of drugs compared with conventional HIPEC. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) combines the benefits of a minimally invasive approach (easier repeat application, lower morbidity, shorter hospital stay) with the pharmacokinetic advantages of intraperitoneal administration (higher intratumoral concentrations, less systemic toxicity) under pressurized vaporization (increased distribution and deeper penetration).19,20 This new drug-delivery technique has been increasingly used in cases of peritoneal metastasis, with some authors reporting promising results both in terms of quality of life (QoL)21 and local disease control.22,23
PIPAC represents an experimental treatment, which is only allowed in controlled clinical trials and is meant to demonstrate an increase in drug effectiveness related to the physical properties of pressure and gas. Scientific evidence supporting PIPAC outside an experimental setting is still lacking.24
In this study, our co-primary endpoint was to investigate the feasibility of PIPAC, in terms of therapeutic efficacy and safety, using cisplatin and doxorubicin in an homogeneous setting of women with platinum-resistant recurrence of ovarian cancer receiving up to two lines of chemotherapy.25 Our secondary endpoint was to determine QoL on the basis of the European Organization for Research and Treatment of Cancer (EORTC) QOL questionnaires (QLQ-C30 and QLQ-OV28), before and after application of the PIPAC cycle.26,27
Methods
From January 2016 to January 2020, patients affected by platinum-resistant recurrence of ovarian tumor, with an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 3, and after one or two previous regimens of chemotherapy, were prospectively recruited at Fondazione Policlinico Universitario “Agostino Gemelli” IRCCS, Rome, Italy. Before admission to the experimental protocol, each patient was discussed in the multidisciplinary tumor board and the indication for PIPAC was decided according to the patients’ clinical conditions and history of disease. A standard preoperative work-up (i.e., chest x-ray, blood tests) was performed before each procedure. Chronic and uncontrolled systemic disorders, bowel obstruction, and/or extraperitoneal metastasis were considered contraindications for PIPAC administration. This study was conducted in accordance with the declaration of Helsinki, was approved by the local Ethical Committee (Prot. N.80, 22/12/2015), and was registered at ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT02735928). Trial management and progress were monitored by an independent data monitoring committee (i.e., Data Collection Facility) and the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement was followed as close as possible.28 All patients signed an informed consent form at least 1 day prior to enrollment.
Surgical Procedure
The PIPAC procedure was performed according to the previously published consensus guidelines of the ISSPP-PIPAC study group.29,30,31 Briefly, after insufflation of 12 mmHg of capnoperitoneum at 37°C, two balloon trocars (Applied Medical®) were placed. Explorative laparoscopy was performed as usual at our institution and the Peritoneal Carcinomatosis Index was determined, according to Fagotti’s score.32,33 Parietal biopsies were taken and ascites were removed. A nebulizer (MIP, Reger Medizintechnik, Rottweil®) was connected to a high-pressure injector (Injektron 82M, MedTron, Saarbruecken®) and was inserted into the abdomen through a trocar. A pressurized aerosol containing cisplatin (Hexal, Barleben) at a dose of 10.5 mg/m2 body surface in 150 mL NaCl 0.9% followed by doxorubicin at a dose of 2.1 mg/m2 body surface in a 50 mL NaCl 0.9% solution was immediately applied via a nebulizer, according to a previous study.34 The system was then kept in a steady state for 30 min (i.e., application time). At the end of the procedure, a peritoneal biopsy was performed, with positioning of a metal landmark clip nearby. The landmark clip was used in subsequent procedures to perform the biopsy at the same point and limit as much as possible as the heterogeneity of the pathologist’s reading on the pathological finding. Toxic aerosol was exhausted over a closed system and the trocars were removed. The PIPAC procedure was repeated after 4–6 weeks until progression or limiting toxicity. Adverse events were graded according to the Memorial Sloan Kettering Cancer Center surgical grading system.35 In the meantime, the QOL questionnaires were administered to assess the QOL of cancer patients after any cycle of PIPAC.
Despite its known limited accuracy in detecting small peritoneal lesions and the involvement of the small bowel/mesentery, the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,36 through contrast-enhanced computed tomography (CT), remains the standard imaging modality in the assessment of PC. However, we defined the clinical benefit rate (CBR) as the percentage of advanced cancer patients who achieved complete response (CR), partial response (PR), or at least 6 months of stable disease (SD) as a result of therapy, according to RECIST 1.1 criteria.37
Since CT shows only limited diagnostic accuracy and underestimates the real extent of peritoneal carcinomatosis,38 we used laparoscopic assessment to complement radiological data and to assess the change in tumor burden. The gold standard for measuring the extent of peritoneal carcinomatosis is the Peritoneal Cancer Index (PCI),39 but in our institution we routinely use Fagotti’s score to evaluate the distribution and volume of the abdominal disease in patients with a first diagnosis of ovarian cancer. Thus, we decided to also apply Fagotti’s scoring method in this population of patients affected by platinum-resistant ovarian cancer.
A priori, as an ancillary report and in addition to the standard chemotherapy treatment response criteria (i.e., RECIST 1.1 criteria), we applied the following laparoscopic definitions for macroscopic response during laparoscopy.
Complete response (CR): Fagotti’s score = 0, with negative histology of at least three peritoneal biopsies of suspect nodules.
Partial response (PR): At least two parameters had a decrease in Fagotti’s score.
Progressive disease (PD): At least one parameter had an increase in Fagotti’s score. The appearance of one or more new lesions is also considered progression.
Stable disease (SD): Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference Fagotti’s score.
However, the laparoscopic evaluation was only used as an ancillary report and no clinical decision was based on it.
Pathological Evaluation
The Peritoneal Regression Grading Score (PRGS) by Solass et al.40 was used to grade the regressive changes in the obtained biopsies and/or surgical specimens. The evaluation of PRGS is defined as follows:
-
Grade 1: Complete response (absence of tumor cells).
-
Grade 2: Major response (major regression features, few residual tumor cells).
-
Grade 3: Minor response (some regressive features but predominance of residual tumor cells).
-
Grade 4: No response (tumor cells without any regressive features).
Herein, an increase in PRGS between the first PIPAC (PIPAC1) and the last PIPAC performed in each patient was determined as iPRGS, as similarly described in a previous paper:41 iPRGS+ in the case of increased PRGS, and iPRGS− in the case of absence of increased PRGS. Mean PRGS was considered when more than one biopsy was performed during the PIPAC procedure.
Statistical Analysis
The co-primary endpoints were to establish the feasibility both in terms of efficacy (i.e., CBR) and safety, while the secondary endpoint was to evaluate QoL during its application. All p-values were two-tailed and a p-value <0.05 was considered statistically significant. The sample size was calculated based on a Simon two-stage design for a phase II study. Based on clinical trials of women with recurrent ovarian cancer undergoing experimental chemotherapy regimens of fewer than two lines of standard chemotherapy and reporting a CBR of 25–60%,42,43,44 we regarded a priori a proportion of patients with a CBR of ≥ 40% as proof of efficacy of PIPAC in this patient population, and of < 20% as insufficient to continue the assessment. Assuming a risk of α = 0.05 (type I error) and β = 0.20 (type II error), we needed to include 36 patients; considering a withdrawal rate of at least 10%, a total of 43 women were enrolled.
Analysis was by intention to treat and was performed using non-parametric tests since data were not normally distributed. Values are given as medians. Survival was modeled in a Kaplan–Meier survival curve. With the term of time to progression (TTP) we mean median time from the ovarian cancer relapse diagnosis to disease progression. Overall survival was defined as the time from the ovarian cancer relapse diagnosis to last follow-up, and QoL was measured using the EORTC QLQ-C30 and QLQ-OV28 questionnaires, validated tools for assessing QoL in cancer patients.24,25 QoL was recorded 1 day before each PIPAC procedure. For evaluation of QoL data, analysis of variance (ANOVA) for repeated measures was used to analyze modifications of QoL measures over time. No imputation was undertaken to deal with missing data at any time-point since there were no missing values.
For statistical analysis, we used NCSS 11.0 software and PASS 15.0 (Power Analysis and Sample Size Software; NCSS, LLC. Kaysville, UT, USA [ncss.com/software/pass]).
Results
Ninety-eight PIPAC procedures were performed in 43 women from January 2016 to January 2020 (Fig. 1). In only three patients, intraperitoneal therapy was not carried out as a result of impossible access to the abdominal cavity due to the extensive and strong adhesions, with a final feasibility rate of 93%. Patients’ median age was 58.5 years (range 33–70 years). Most patients (88.4%) were affected by ovarian serous carcinoma (36 woman had a high-grade serous carcinoma and two patients had a low-grade serous carcinoma), four women were affected by clear cell cancers and one women was affected by mucinous carcinoma. All patients received fewer than two platinum-based chemotherapy regimens after primary or interval debulking surgery, with a median platinum-free interval of 4 months (range 0–6) (Table 1). At the time of laparoscopy for the PIPAC procedure, the median Fagotti’s score was 10 (range 8–12) and ascites was documented in 13 patients (30.2%). Almost half of the patients underwent three or more cycles of PIPAC therapy (18 patients, 45.0%), while 13 (32.5%) and 9 (22.5%) patients were subjected to only one and two cycles of PIPAC, respectively, due to disease progression documented with a radiological instrumental examination performed for worsening of clinical conditions.
The median operative time was 145 min (range 90–295 min), median estimated blood loss was 5 mL (range 0–10 mL), and the median length of hospital stay was 2 days (range 1–10 days). Early postoperative complications were documented during 8 of the 98 total procedures (8.2%). G2 complications occurred in six patients, including two patients with bowel obstruction that was managed with intravenous hydration and parenteral nutrition, one case of anemia treated with a blood transfusion, two patients with systemic infections, and one patient with abdominal collection that required antibiotic therapy. Two (2%) patients had G3 complications, both with intestinal perforation during open laparoscopic entry, requiring operative laparotomy to repair a lesion and antibiotic therapy during postoperative hospital stay. Thirty-day mortality was not documented. During PIPAC therapy, sclerosis of peritoneal nodules was observed, as well as scarring of the visceral and parietal peritoneum (Table 2).
Corresponding histological specimens taken during the first, second, and third PIPAC application demonstrated regressive tumor changes, fibrosis, and acute and chronic inflammation (Fig. 2). During the PIPAC treatment period, some variations in the PRGS were observed comparing the value assessed on biopsies performed during the first PIPAC procedure and the last biopsy for each patient. In detail, 19 patients showed an increase in PRGS and 8 patients did not show an increase; all 8 women show minor response (grade 3) and no patients showed a decrease in the PRGS. Otherwise, no macroscopic variations of carcinosis were observed, as described by Fagotti’s scores, with respect to histological findings (data not shown).
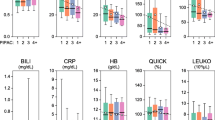
EORTC QLQ-30 symptom scores did not show any statistical differences, even if nausea/vomiting, appetite loss, dyspnea, emotional functioning, and constipation appeared to slightly decrease during therapy. In addition, physical, cognitive, fatigue, and pain scores increased during therapy. On the QLQ-OV28 symptom score, we also did not observe any statistical difference. However, some abdominal symptoms seemed to slightly increase and, on the other hand, other chemotherapy adverse effects, such as body image and hair loss, appeared to improve during therapy (Electronic Supplementary Tables 1 and 2).
Post-relapse survival and overall survival are shown in Figs. 3 and 4. The median duration of follow-up was 30 months. With a CBR of 82%, the median time from ovarian cancer relapse diagnosis to disease progression (time to progression [TTP]) was 12 months (95% confidence interval [CI] 6.483–17.517), documented by radiological examinations, while the median time from the last PIPAC procedure to disease progression was 1 month (0–6).
Median overall survival was 27 months (95% CI 20.337–33.663). Twenty-eight patients (70%) died as a result of multi-organ failure seen with disease progression at the time of last follow-up.
Discussion
Large population-based studies have shown that patients with platinum-resistant recurrence of ovarian cancer have poor prognosis, with a median overall survival of 10–12 months.46 New treatment modalities are desperately needed and PIPAC-directed therapy has emerged as an option in this subset of women. To our knowledge, this is the first study that tests the feasibility of PIPAC, in terms of efficacy and safety, in an homogeneous series of platinum-resistant patients who underwent a maximum of two lines of previous chemotherapy treatment (ClinicalTrials.gov identifier: NCT02735928). Our study shows that PIPAC with intraperitoneal cisplatin and doxorubicin is active and well tolerated in women with platinum-resistant ovarian cancer, with 82% of patients achieving a CBR.
Regarding the recent literature data on PIPAC,47,48,49,50,51 the strength of this manuscript lies in the fact that it is the first phase II trial on women with the same advanced disease (i.e., ovarian cancer), the biological characteristics (i.e., platinum-resistant), and the same number of previous lines of chemotherapies administered. Moreover, while the trial was ongoing, although different authors49,50 performed dose-escalation studies that highlighted that PIPAC could be performed at the highest dosage, we decided to maintain the exact dosage according to Tempfer at al.34 to guarantee more homogeneous results. Moreover, the reason for the limited cycle of PIPAC in 55% of patients was due to disease progression documented with a radiological instrumental examination or for worsening of clinical conditions. Indeed, we should consider the unfavorable setting of women in this trial (platinum-resistant after only one to two lines of chemotherapy) with respect to other experiences.22,47,48
Our results agree with the preliminary, and still unpublished, results of the Indian phase II study that was presented in poster format at ASCO in 2022, in which PIPAC was shown to be safe and feasible for patients with unresectable platinum-resistant ovarian cancer.47 Somashekhar et al.48 have already published data regarding the application of PIPAC in patients affected by gynecological and gastrointestinal tumors with end-stage peritoneal metastasis. They showed a promising response, a good tolerance profile, and QoL after three cycles of PIPAC in comparison with six cycles of systemic chemotherapy in this inhomogeneous cohort of patients, using the EORTC QLQ-C30 (version 3.0) questionnaire.
Of note, overall QoL scores for global physical health scores do not decrease during therapy. An objective minor tumor response and no tumor response with SD was documented in 29.6% and 70.4% of patients, respectively, after two PIPAC-directed treatments. The median overall survival after the first diagnosis was 27.0 months, while time to progression after the first PIPAC procedure was 12 months. These results agree well with the recently presented experiences from German centers and are consistent with previous experience with PIPAC in patients with platinum-resistant recurrence.52
Until now, anecdotal evidence12 and retrospective case series15 demonstrated objective tumor response and histological tumor regression in ovarian cancer, with acceptable local and systemic toxicity. This is the first prospective report that seems to confirm these results in a homogeneous clinical subset of patients.
Our data confirm that PIPAC is safe and well tolerated in women with platinum-resistant ovarian cancer, even if applied without concomitant cytoreductive surgery. Moreover, bearing in mind that the administration of some drugs (i.e., bevacizumab) is associated with a similar bowel perforation rate without surgery, the PIPAC would not replace them but would integrate itself with the timing of other drugs administered in this subset of patients.
Of note, most women in our trial were pretreated with a median number of one prior chemotherapy regimens, indicating that PIPAC may be able to overcome platinum resistance at least in a fraction of women. This may be related to the high local chemotherapy concentrations achieved by the intra-abdominal application, and/or by the better uptake of the drug induced by the peritoneal vasodilation caused by the hypercapnic intraperitoneal environment of the pneumoperitoneum.17 Another factor may be the chemical peritonitis induced by intra-abdominal application of the cytotoxic drugs themselves.53 A potential beneficial aspect of PIPAC compared with systemic cytotoxic chemotherapy may be its adverse effect profile. In order to objectify the impact of PIPAC on the QoL of study participants, we have systematically measured QoL scores using the EORTC QLQ-30 and QLQ-OV28 questionnaires. It is of note that gastrointestinal scores such as those for nausea/vomiting, appetite loss, and constipation slightly decreased during therapy, which could indicate that PIPAC stabilizes peritoneal carcinosis and leads to an improvement of bowel function. In addition, physical, cognitive, and emotional scores improved and fatigue scores decreased during therapy, supporting the notion that the women experienced a clinical benefit from PIPAC. Notorious systemic adverse effects of chemotherapy, such as alopecia, peripheral neurotoxicity, nausea, and myelosuppression, did not occur with PIPAC.
This phase II trial has some limitations. First, patients were highly selected; for example, women with liver and lung metastases were excluded, and it is possible that patients with the best prognosis were thus selected. However, our main goal was to report the feasibility of procedures in an homogeneous setting of women. Moreover, although, as a monocentric trial, a weakness could lie in the lack of variation of the surgical experience in different institutes, at the same time it offers the surgical background of a single center where surgical care and laparoscopic evaluation are standardized, overcoming any difference such as preoperative patient selection, surgical strategies, and postoperative management.
Second, the small number of patients in this phase II trial may obscure rare adverse events and toxicities. Furthermore, we have no long-term follow-up data and cannot rule out late adverse events such as bowel sclerosis. Third, although no clinical decision was performed based on Fagotti’s score, since it has been developed as a staging tool for peritoneal carcinomatosis and not as a measure of therapy response, we did not observe any regressions in terms of peritoneal dissemination, but this evaluation could be incorrect. Lastly, pathological response assessment may be inaccurate because it is difficult to differentiate between scars and vital tumor tissue, and, even if we use a landmark for peritoneal biopsy, the heterogeneity of the peritoneal carcinosis could not mirror the response to chemotherapy.
In summary, PIPAC seems to play an active role in platinum-resistant ovarian cancer recurrence, is easy to perform, and is well tolerated if strict selection of patients was performed. PIPAC is not associated with a decrease in QoL. Whether or not PIPAC is a clinically meaningful therapeutic option in the setting of palliative ovarian cancer treatment remains to be seen.
Further comparative clinical trials testing the efficacy of PIPAC versus, or in addition to, systemic chemotherapy are warranted, thus making it possible to treat women with extra-abdominal disease. Indeed, this paper is only the first mandatory step to pave the subsequent scientific trials, trying to extend the applications of PIPAC with or without concomitant chemotherapy in different subsets of women with ovarian cancer, choosing the right dosage and timing.
Thus, we consider that our study may be hypothesis-generating since the potential role of other chemotherapy compounds such as taxanes, topotecan, gemcitabine, and/or nanoparticles should be investigated in PIPAC protocols in this or different subsets of patients. Indeed, since the platinum-resistant nature of the relapse, these agents may be more effective and PIPAC should be intended as a new different drug delivery system.
Finally, combining cytotoxic agents with bevacizumab and/or its role in the poly(ADP-ribose) polymerase (PARP) inhibitor era with or without BRCA mutation may be an attractive option for future PIPAC trial designs.
References
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–88.
Du Bois A, Vergote I, Ferron G, et al. Randomized controlled phase III study evaluating the impact of secondary cytoreductive surgery in recurrent ovarian cancer: AGO DESKTOP III/ENGOT-ov20. J Clin Oncol 2020;38:abstract no. 6000.
Petrillo M, Fagotti A, Ferrandina G, et al. Ovarian cancer patients with localized relapse: clinical outcome and prognostic factors. Gynecol Oncol. 2013;131:36–41.
Salani R, Santillan A, Zahurak ML, et al. Secondary cytoreductive surgery for localized, recurrent epithelial ovarian cancer: analysis of prognostic factors and survival outcome. Cancer. 2007;109:685–91.
Lee CK, Lord S, Grunewald T, et al. Impact of secondary cytoreductive surgery on survival in patients with platinum sensitive recurrent ovarian cancer: analysis of the CALYPSO trial. Gynecol Oncol. 2015;136(1):18–24.
Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106(9):1933–9.
van de Laar R, Zusterzeel PL, Van Gorp T, et al. Cytoreductive surgery followed by chemotherapy versus chemotherapy alone for recurrent platinum-sensitive epithelial ovarian cancer (SOCceR trial): a multicenter randomized controlled study. BMC Cancer. 2014;14:22.
Eriksson AGZ, Graul A, Yu MC, et al. Minimal access surgery compared to laparotomy for secondary surgical cytoreduction in patients with recurrent ovarian carcinoma: Perioperative and oncologic outcomes. Gynecol Oncol. 2017;146(2):263–7.
Gallotta V, Conte C, Giudice MT, et al. Secondary laparoscopic cytoreduction in recurrent ovarian cancer: a large, single-institution experience. J Minim Invasive Gynecol. 2018;25(4):644–50.
National Comprehensive Cancer Network (NCCN) Clinical practice guidelines in ovarian cancer. Version 2.2023.
Petrillo M, Pedone Anchora L, Tortorella L, et al. Secondary cytoreductive surgery in patients with isolated platinum-resistant recurrent ovarian Cancer: a retrospective analysis. Gynecol Oncol. 2014;134(2):257–61.
Musella A, Marchetti C, Palaia I, et al. Secondary cytoreduction in platinum-resistant recurrent ovarian cancer: a single-institution experience. Ann Surg Oncol. 2015;22:4211–6.
Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4:277–83.
Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–92.
Hotouras A, Desai D, Bhan C, Murphy J, Lampe B, Sugarbaker PH. Heated IntraPEritoneal chemotherapy (HIPEC) for patients with recurrent ovarian cancer: a systematic literature review. Int J Gynecol Cancer. 2016;26(4):661–70.
Fagotti A, Costantini B, Gallotta V, et al. Minimally invasive secondary cytoreduction plus HIPEC versus open surgery plus HIPEC in isolated relapse from ovarian cancer: a retrospective cohort study on perioperative outcomes. J Minim Invasive Gynecol. 2015;22(3):428–32.
Fagotti A, Petrillo M, Costantini B, et al. Minimally invasive secondary cytoreduction plus HIPEC for recurrent ovarian cancer: a case series. Gynecol Oncol. 2014;132(2):303–6.
Raspagliesi F, Kusamura S, Campos Torres JC, et al. Cytoreduction combined with intraperitoneal hyperthermic perfusion chemotherapy in advanced/recurrent ovarian cancer patients: the experience of National Cancer institute of Milan. Eur J Surg Oncol. 2006;32(6):671–5.
Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol. 2016;14:128.
Solass W, Kerb R, Mürdter T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21:553–9.
Odendahl K, Solass W, Demtröder C, et al. Quality of life of patients with end-stage peritoneal metastasis treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Eur J Surg Oncol. 2015;41:1379–85.
Tempfer CB, Winnekendonk G, Solass W, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: a phase 2 study. Gynecol Oncol. 2015;137(2):223–8.
Tempfer CB, Solass W, Reymond MA. Pressurized intraperitoneal chemotherapy (PIPAC) in women with gynecologic malignancies: a review. Wien Med Wochenschr. 2014;164(23–24):519–28.
Dueckelmann AM, Fink D, Harter P, et al. The use of PIPAC (pressurized intraperitoneal aerosol chemotherapy) in gynecological oncology: a statement by the German “Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR)”, the Swiss and Austrian AGO, and the North-Eastern German Society of Gynaecologic Oncology. Arch Gynecol Obstet. 2018;297:837–46.
Solass W, Hetzel A, Nadiradze G, Sagynaliev E, Reymond MA. Intraoperative intraperitonal drug delivery using a nebulizer: rationale and pharmacokinetic results. Surg Endosc. 2012;26(7):1849–55.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Greimel E, Bottomley A, Cull A, et al. EORTC Quality of Life Group and the Quality of Life Unit. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-OV28) in assessing the quality of life of patients with ovarian cancer. Eur J Cancer. 2003;39(10):1402–8.
Standard Protocol Items: Recommendations for Interventional Trials. https://www.spirit-statement.org/
Hübner M, Alyami M, Villeneuve L, et al. ISSPP PIPAC study group. Consensus guidelines for pressurized intraperitoneal aerosol chemotherapy: technical aspects and treatment protocols. Eur J Surg Oncol. 2022;48(4):789–94.
Sgarbura O, Villeneuve L, Alyami M, et al. ISSPP PIPAC study. Group. Current practice of pressurized intraperitoneal aerosol chemotherapy (PIPAC): still standardized or on the verge of diversification? Eur J Surg Oncol. 2021;47(1):149–56.
Alyami M, Sgarbura O, Khomyakov V, et al. Standardizing training for pressurized intraperitoneal aerosol chemotherapy. Eur J Surg Oncol. 2020;46(12):2270–5.
Fagotti A, Ferrandina G, Fanfani F, et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol. 2006;13:1156–61.
Chéreau E, Ballester M, Selle F, Cortez A, Daraï E, Rouzier R. Comparison of peritoneal carcinomatosis scoring methods in predicting resectability and prognosis in advanced ovarian cancer. Am J Obstet Gynecol. 2010;202(2):178.e1-178.e10.
Tempfer CB, Giger-Pabst U, Seebacher V, Petersen M, Dogan A, Rezniczek GA. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol Oncol. 2018;150(1):23–30.
Strong VE, Selby LV, Sovel M, et al. Development and assessment of Memorial Sloan Kettering Cancer Center’s Surgical Secondary Events grading system. Ann Surg Oncol. 2015;22(4):1061–7.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Am Delgado A, Guddati AK. Clinical endpoints in oncology—a primer. Am J Cancer Res. 2021;11(4):1121–31.
Ferrandina G, Sallustio G, Fagotti A, et al. Role of CT scan-based and clinical evaluation in the preoperative prediction of optimal cytoreduction in advanced ovarian cancer: a prospective trial. Br J Cancer. 2009;101(7):1066–73.
Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In: PH Sugarbaker, editor. Peritoneal carcinomatosis: principles of management. Boston: Springer; 1996. p. 359–74.
Solass W, Sempoux C, Detlefsen S, Carr NJ, Bibeau F. Peritoneal sampling and histological assessament of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum. 2016;1(2):99–107.
Benzerdjeb N, Durieux E, Tantot J, et al. Prognostic impact of combined progression index based on peritoneal grading regression score and peritoneal cytology in peritoneal metastasis. Histopathology. 2020;77(4):548–59.
Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA Study. J Clin Oncol. 2023;41(13):2436–45.
Musacchio L, Cicala CM, Salutari V, et al. Preclinical and clinical evidence of lurbinectedin in ovarian cancer: current status and future perspectives. Front Oncol. 2022;12:831612.
Gaillard S, Oaknin A, Ray-Coquard I, et al. Lurbinectedin versus pegylated liposomal doxorubicin or topotecan in patients with platinum-resistant ovarian cancer: a multicenter, randomized, controlled, open-label phase 3 study (CORAIL). Gynecol Oncol. 2021;163(2):237–45.
https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed 9 Mar 2018.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Somashekhar SP, Ashwin KR, Rauthan A, et al. Pressurized intraPeritoneal aerosol chemotherapy vs. intravenous chemotherapy for unresectable peritoneal metastases secondary to platinum resistant ovarian cancer—study protocol for a randomized control trial. Pleura Peritoneum. 2019;4(1):20180111.
Somashekhar SP, Ashwin KR, Rauthan CA, Rohit KC. Randomized control trial comparing quality of life of patients with end-stage peritoneal metastasis treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) and intravenous chemotherapy. Pleura Peritoneum. 2018;3(3):20180110.
Robella M, De Simone M, Berchialla P, et al. A phase I dose escalation study of oxaliplatin, cisplatin and doxorubicin applied as PIPAC in patients with peritoneal carcinomatosis. Cancers (Basel). 2021;13(5):1060.
Somashekhar S, Kumar R, Kapoor P, et al. EP290/#660 First report of clinical outcomes with escalated doses of cisplatin and doxorubicin in PIPAC for peritoneal carcinomatosis of epithelial ovarian cancer. Int J Gynecolo Cancer. 2022;32:A170.
Taliento C, Restaino S, Scutiero G, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in patients with ovarian cancer: a systematic review. Eur J Surg Oncol. 2023;49(12):107250.
Horvath P, Yurttas C, Baur I, et al. Current medical care situation of patients in Germany undergoing pressurized intraperitoneal aerosol chemotherapy (PIPAC). Cancers. 2022;14(6):1443.
Lu Z, Wang J, Wientjes MG, Au JL. Intraperitoneal therapy for peritoneal cancer. Future Oncol. 2010;6(10):1625–41.
Funding
Open access funding provided by Università degli Studi di Udine within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Giuseppe Vizzielli, Maria Teresa Giudice, Federica Nardelli, Barbara Costantini, Vanda Salutari, Frediano Socrate Inzani, Gian Franco Zannoni, Vito Chiantera, Andrea Di Giorgio, Fabio Pacelli, Anna Fagotti, and Giovanni Scambia declare they have no conflicts of interest in relation to this current work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vizzielli, G., Giudice, M.T., Nardelli, F. et al. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) Applied to Platinum-Resistant Recurrence of Ovarian Tumor: A Single-Institution Experience (ID: PARROT Trial). Ann Surg Oncol 31, 1207–1216 (2024). https://doi.org/10.1245/s10434-023-14648-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14648-0