Abstract
Purpose
In sentinel node-positive (SN+ve) melanoma patients, active surveillance with regular ultrasound examination of the node field has become standard, rather than completion lymph node dissection (CLND). A proportion of these patients now receive adjuvant systemic therapy and have routine cross-sectional imaging (computed tomography [CT] or positron emission tomography [PET]/CT). The role of concurrent ultrasound (US) surveillance in these patients is unclear. The purpose of our study was to describe the modality of detection of nodal recurrence in SN+ve node fields.
Methods
SN+ve melanoma patients who did not undergo CLND treated at a single institution from January 1, 2016 to December 31, 2020 were included.
Results
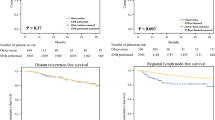
A total of 225 SN+ve patients with a median follow-up of 23 months were included. Of these, 119 (53%) received adjuvant systemic therapy. Eighty (36%) developed a recurrence at any site; 24 (11%) recurred first in the SN+ve field, of which 12 (5%) were confirmed node field recurrence only at 2 months follow-up. The nodal recurrences were first detected by ultrasound in seven (3%), CT in seven (3%), and PET/CT in seven (3%) patients. All nodal recurrences evident on US were also evident on PET/CT and vice versa.
Conclusions
The high rate of recurrences outside the node field and the identification of all US-detected nodal recurrences on concurrent cross-sectional imaging modalities suggest that routine concurrent ultrasound surveillance of the node-positive field may be unnecessary for SN+ve melanoma patients having routine cross-sectional imaging.
Similar content being viewed by others
Contemporary surgical management of primary cutaneous melanomas in patients at high risk of regional lymph node metastasis involves wide local excision and sentinel node (SN) biopsy (SNB). If a SN is found to contain metastatic melanoma, active surveillance of the node field is currently widely accepted. Previously, completion lymph node dissection (CLND) was recommended for SN-positive (SN+ve) melanoma patients. However, two seminal randomized trials, MSLT-II and DeCOG-SLT, failed to demonstrate a melanoma-specific survival (MSS) advantage from immediate CLND in SN+ve patients compared with nodal surveillance and delayed therapeutic lymph node dissection (TLND) for node field recurrence only.1,2 Where CLND was omitted in these trials, ultrasound surveillance of the SN+ve node field was mandated.1,2 Disease-free survival was improved for the immediate CLND group, but this came at the cost of a significantly higher risk of morbidity. Thus, CLND is no longer performed routinely.
Since these trials commenced recruiting patients in 2004 and 2006 respectively, systemic therapy options for melanoma patients have been revolutionized. Early adjuvant systemic therapy trials mandated CLND for SN+ve patients; however, this is no longer the case. Many of these patients are now offered adjuvant systemic therapy with either immune checkpoint inhibitors or BRAF/MEK inhibitors, both of which have been shown to significantly improve recurrence-free survival (RFS) compared with placebo (or ipilimumab).3,4,5
In patients who receive adjuvant systemic therapy, frequent cross-sectional imaging is usually performed, commonly at 3-monthly or 4-monthly intervals for the first 2 years and with decreasing frequency thereafter. Some patients who only have active surveillance undergo cross-sectional imaging at the same intervals as those receiving adjuvant therapy. Adjuvant therapy trials did not mandate ultrasound surveillance of the SN+ve field, despite US previously having been shown to be superior to computed tomography [CT], positron emission tomography [PET], and PET/CT for the early detection of regional lymph node metastases.3,4,5,6 Consequently, routine US often is not part of the surveillance imaging schedule, particularly when medical oncologists are undertaking the follow-up. Ultrasound is still commonly performed in routine practice for regional node surveillance of patients not receiving systemic adjuvant therapy and undergoing follow-up with their surgeon and/or referring dermatologist or primary care physician. This is frequently in conjunction with less frequent whole-body cross-sectional imaging.
Whether there is still a place for US surveillance of the regional lymph nodes of SN+ve patients in the modern era is unclear, particularly given the frequent use of whole-body cross-sectional imaging. To address this question, the purpose of this study was to describe the sites of recurrence and modality of their detection in SN+ve melanoma patients.
Methods
Study Design
For this retrospective study, data were extracted from a prospectively-maintained database for patients with primary cutaneous melanoma and a positive SN treated at a large Australian melanoma treatment centre (Melanoma Institute Australia [MIA]) from January 1, 2016 to December 31, 2020 and who did not have CLND. Patients were included if they had a clear baseline scan at the time of their SNB or if they did not have a baseline scan, a minimum 2-month interval from the date of SNB to the detection of nodal recurrence in the SN field. Exclusion criteria were a previous or subsequent higher-stage melanoma or concurrent in-transit metastases. Written consent for the use of their data had been obtained from all patients. The study was approved by the MIA Research Committee (MIA2022/447) under Human Research Ethics Committee (HREC) Sydney Local Health District Protocol No X15-0311 & 2019/ETH06854.
Outcomes
The following variables were collected for the whole cohort: sex, age at time of SNB, melanoma subtype, location of primary tumor (head or neck, upper limb, lower limb, trunk), Breslow thickness (mm), ulceration status, mitotic rate, microsatellites, extracapsular extension, site(s) and if more than one site of excised SN and total number of excised LNs (SNs and non-SNs), total number of positive SNs, AJCC stage (8th edition) at time of diagnosis, adjuvant therapy (and type) if administered, duration of follow-up, and status at last follow-up.
The primary endpoint was modality of detection (patient, clinician, US, CT, or PET/CT) of nodal recurrence, as the first site of recurrence, in a node field from which a positive SN had previously been removed. Nodal recurrence was defined as node field recurrence only or node field and other site(s) (e.g., in-transit or distant metastasis), with the latter defined as any further recurrence detected within 2 months of the nodal recurrence. Node field recurrence and other sites was defined as radiologically evident metastatic disease with or without biopsy confirmation.
Secondary endpoints included whether imaging performed within 2 months of the nodal recurrence demonstrated the node recurrence, days between the scans (US, PET/CT, and/or CT), time to nodal recurrence (from SNB to nodal recurrence in SN field), adjuvant therapy at the time of nodal recurrence, and if recurrence occurred while on adjuvant therapy whether this led to a change in treatment. If the patient recurred in the node field as well as elsewhere concurrently, the burden of disease was recorded as oligometastatic disease (1–3 site(s)) or high-volume disease (≥4 sites), and sites of disease were documented.
Details of surgical treatment of nodal recurrence were collected, including whether this was performed in patients with node field recurrence only or node field and other site(s) recurrences, type of surgery (selective excision of involved nodes or TLND), number of involved nodes, largest diameter of the excised nodal metastasis, and presence or absence of extracapsular extension. The maximum diameter of each excised nodal metastasis was further stratified according to the imaging modality by which it was first detected. Lastly, the size of the nodal metastasis (the short axis of the lymph node metastasis), measured on the imaging modality, which detected the nodal recurrence, was documented.
Descriptive Analysis
Key summary statistics were derived for patients’ characteristics and clinicopathologic features. All percentages were calculated as relative to the whole cohort. To explore associations between each variable and different types of recurrences, P-values were obtained from Kruskal-Wallis rank sum tests for continuous variables and Fisher’s exact tests for categorical variables. Missing values were not considered when computing the Fisher’s exact test.
Results
Patient Population, Imaging, and Follow-Up
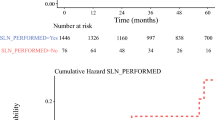
Between 2016 and 2020, 225 SN+ve patients in whom CLND was omitted underwent surveillance as recommended by their surgeon and/or medical oncologist (Table 1). Typically, the surveillance included clinical examination and scans. The scans (US, PET/CT, and/or CT), and their frequency differed, depending on perceived risk of recurrence, individual surgeon, and/or medical oncologist preference or clinical trial protocol. Of the 225 SN+ve patients, 119 (53%) received adjuvant systemic therapy; 27 (12%), 35 (16%), 55 (24%), and two (1%) received adjuvant therapy for stage IIIA, IIIB, IIIC, and IIID disease, respectively. The median follow-up was 23 months (interquartile range [IQR] 12–34).
Recurrence
At last follow-up, 12 patients (5%) had developed an initial first recurrence only in the draining lymph node field, 12 patients (5%) had recurred at multiple sites, including the SN+ve field, 56 (25%) had recurred only outside the node field, and 145 (64%) were recurrence-free. The descriptive tests showed that greater age, greater Breslow thickness, presence of ulceration, higher mitotic rate, presence of microsatellites, and higher AJCC stage were associated with recurrence (Table 1). The median time to nodal recurrence was 10 months (IQR 5–15; Table 2). Six (3%) of the 24 nodal recurrences were diagnosed whilst the patient was receiving adjuvant therapy.
Detection of Nodal Recurrence
Nodal metastases were detected as a first site of recurrence in 24 patients. These were first detected by imaging in 21 patients (9%), by the patient in one (0.5%), and by the clinician at the physical examination in two (1%). The imaging modality first detecting the nodal recurrence was ultrasound in seven patients (3%), CT in seven patients (3%), and PET/CT in seven patients (3%). No method of detection detected a particular site of nodal recurrence better any other site (data not shown). In the seven patients who had nodal recurrence detected by US, the disease was node field recurrence only in five. There was no evidence of any site being more reliable for early detection by US (data not shown). Of all 24 patients found to have node field recurrence, it was node field recurrence only in 12 (5%); the remaining 12 patients (5%) showing concurrent disease at other sites. Of those who first recurred in the draining node field, 19 patients had more than one scan performed within a median of 18 days of the nodal recurrence. All nodal recurrences detected by US also were evident on PET/CT and all but one on CT. In one (0.5%) of the 19 patients, the nodal metastasis was not visible on all concurrent imaging modalities, as the lesion was visible on US and PET/CT, but not evident on CT.
Pattern of Nodal Recurrence
As shown in Table 1, in 56 patients (25%) recurrence at any site except the node field and in 24 (11%) recurrence was in the SN field. In the patients who recurred in the SN field, half also had recurred at other sites at the time of recurrence detection (Table 3). These node field and other site(s) recurrences were “high-volume” disease, defined as at four or more sites in eight patients (4%).
Discussion
In this study of patients who were SN+ve and did not have a CLND, only a minority (5%) developed node field recurrence only in the SN+ve field, whereas others recurred both in the SN+ve node field and other sites, e.g., in-transit or distant metastasis (5%), or outside the SN+ve field (25%). For patients who recurred in the SN+ve field, this study demonstrated that all nodal recurrences were detectable on both US and PET/CT scans with a median scan interval of 18 days. Our data suggest that US may not need to be conducted concurrently if patients are having regular cross-sectional imaging.
Many of our results are consistent with previous studies. Montgomery et al. assessed 109 SN+ve patients in whom CLND was omitted, detecting 13 (12%) node field recurrences after a median follow-up of 15 months.7 In Montgomery’s study, where 57% received adjuvant therapy, only 24% experienced disease recurrence, but they had a significantly shorter median follow-up interval of only 15 months compared with 23 months in our study.
Bartlett et al. similarly examined 370 SN+ve patients in whom CLND was omitted. After a median follow-up of 33 months, 158 (43%) developed recurrences, of which 13% were node-only, 12% local, satellite and/or in-transit, 4% combined (local, satellite and/or in-transit with nodal involvement), and 14% systemic.8 Overall, the recurrence rate of 43 % in that study was higher than in our study where 36% recurred at any site, which may partly be explained by a much lower rate of adjuvant systemic therapy in Bartlett’s study (only 6% had adjuvant systemic therapy compared to 53% of our cohort). Another reason might be the longer duration of follow-up in their study compared with ours (median 33 vs. 23 months). In Bartlett’s study, imaging with CT, PET/CT, and/or US was done at the discretion of the attending physician. Most of the recurrences were detected by cross-sectional imaging (40%) with only 14% detected by node field US. This study did not, however, assess the detection of nodal recurrences by concurrent imaging results.
Node field recurrences in our cohort of SN+ve patients were detected at a median of 10 months after SNB, consistent with previous reports.3,4,5,9 The highest risk of recurrence in all previously reported studies and in the present study is within the first 2 years after initial melanoma diagnosis, justifying more intense surveillance during this period.1,10,11,12
The Breslow thickness, presence of ulceration and microsatellites, as well as increased mitotic rate are well-known risk factors for SN positivity.13,14 These factors also were associated with both nodal and other recurrences in our patient cohort (Table 1).
Detection of nodal recurrences will be determined by the test(s) that is performed and order hereof. The high rate of concurrent node field and other site(s) disease at the time of detection of nodal recurrences and/or recurrences at any site except node field in our cohort, highlights the importance of cross-sectional imaging in this patient group. Given that both PET/CT and US detected the nodal recurrences in all patients who underwent both scans, use of ultrasound in conjunction with cross-sectional imaging may be unnecessary if patients are followed-up by cross-sectional imaging. Ultrasound provides an imaging modality without radiation exposure, which is of particular importance in young patients with stage IIIA disease, who are at lower risk of recurrence. The type and frequency of surveillance imaging for SN+ve patients vary considerably, depending on clinician, imaging availability, and cost to the institution and patient and the optimal frequency and duration of imaging remain to be determined. Regardless of imaging modality, the potential risks of false-positive results, patient anxiety, and, for PET/CT and CT, radiation exposure must be considered.10
Inconsistency of type of imaging and intervals hereof is a limitation in our study. Only the scans performed within 2 months after detection of nodal recurrence in a SN+ve field were assessed in our study. Total number of scans, false-positive findings, and incidental findings were not addressed. Another limitation is the retrospective nature of the study and its limited size (225 patients), with only 24 recurrences in a node field. Furthermore, the follow-up interval was relatively short with a median of only 23 months, and there was uncertainty about blinding of the reporting sonographer, radiologist, and nuclear physician regarding reporting of concurrently or previously performed scans in the patients who developed SN+ve node field recurrence, which may have affected the reporting of recurrence detection.
Despite limitations, we report a cohort of SN+ve patients, treated in the current era, where CLND was omitted and where cross-sectional imaging was frequently used. This particularly distinguishes it from previous similar studies and increases the clinical applicability of our results.
Conclusions
SN+ve patients for whom CLND was omitted are at risk of recurrences not only in the SN field but also at other sites (other locoregional recurrence and/or distant metastases). Moreover, nodal recurrences are often part of a multisite disease progression. These tend to occur with the highest incidence within the first 2 years after diagnosis and are best detected on cross-sectional imaging. Despite previously reported greater sensitivity of US for nodal metastasis detection, the higher rate of recurrences outside the node field and the comparable detection rate of nodal recurrences by cross-sectional imaging modalities suggests that routine, concurrent ultrasound surveillance may be unnecessary for SN+ve melanoma patients, irrespective of whether the patient receives adjuvant therapy, if regular cross-sectional imaging is performed, specifically PET/CT.
References
Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–22.
Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–67.
Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–801.
Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–35.
Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–23.
Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103(2):129–42.
Montgomery KB, Correya TA, Broman KK. Real-world adherence to nodal surveillance for sentinel lymph node-positive melanoma. Ann Surg Oncol. 2022;29(9):5961–8. https://doi.org/10.1245/s10434-022-11839-z.
Bartlett EK, Lee AY, Spanheimer PM, et al. Nodal and systemic recurrence following observation of a positive sentinel lymph node in melanoma. Br J Surg. 2020;107(11):1480–8.
Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–92.
Helvind NM, Aros Mardones CA, Holmich LR, et al. Routine PET-CT scans provide early and accurate recurrence detection in asymptomatic stage IIB-III melanoma patients. Eur J Surg Oncol. 2021;47(12):3020–7.
Nijhuis AAG, Dieng M, Khanna N, et al. False-positive results and incidental findings with annual CT or PET/CT surveillance in asymptomatic patients with resected stage III melanoma. Ann Surg Oncol. 2019;26(6):1860–8. https://doi.org/10.1245/s10434-019-07311-0.
Thompson JF, Hyngstrom J, Caracò C, Zager JS, Jahkola T, Bowles TL, Pennacchioli E, Beitsch PD, Hoekstra HJ, Moncrieff M, Ingvar C, van Akkooi A, Sabel MS, Levine EA, Agnese D, Henderson M, Dummer R, Neves R, Rossi CR, Kane JM 3rd, Trocha S, Wright F, Byrd DR, Matter M, Hsueh EC, MacKenzie-Ross A, Kelley M, Terheyden P, Huston TL, Wayne JD, Neuman H, Smithers BM, Ariyan CE, Desai D, Gershenwald JE, Schneebaum S, Gesierich A, Jacobs LK, Lewis JM, McMasters KM, O’Donoghue C, van der Westhuizen A, Sardi A, Barth R, Barone R, McKinnon JG, Slingluff CL, Farma JM, Schultz E, Scheri RP, Vidal-Sicart S, Molina M, Testori AAE, Foshag LJ, Van Kreuningen L, Wang HJ, Sim MS, Scolyer RA, Elashoff DE, Cochran AJ, Faries MB. Therapeutic value of sentinel lymph node biopsy in patients with melanoma: a randomized clinical trial. JAMA Surg. 2022;157:835–42.
Lo SNMJ, Scolyer RA, Haydu LE, Stretch JR, Saw RPM, Nieweg OE, Shannon KF, Spillane AJ, Ch’ng S, Mann GJ, Gershenwald JE, Thompson JF, Varey AHR. Improved risk prediction calculator for sentinel node positivity in patients with melanoma: the Melanoma Institute Australia Nomogram. J Clin Oncol. 2020;38(24):2719–27.
Niebling MG, Haydu LE, Lo SN, et al. The prognostic significance of microsatellites in cutaneous melanoma. Mod Pathol. 2020;33(7):1369–79.
Acknowledgement
The authors thank the patients who gave permission for their data to be entered into the MIA database and used for research purposes as well as Hazel Burke for data collection. Assistance from colleagues at Melanoma Institute Australia and the Royal Prince Alfred Hospital is gratefully acknowledged. This work was supported by the Cameron Family Foundation.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gjorup, C.A., Woodford, R., Li, I. et al. Role of Concurrent Ultrasound Surveillance of Sentinel Node-Positive Node Fields in Melanoma Patients Having Routine Cross-Sectional Imaging. Ann Surg Oncol 31, 1857–1864 (2024). https://doi.org/10.1245/s10434-023-14526-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14526-9