Abstract
Purpose
This large-scale, multicenter, retrospective observational study aimed to evaluate the clinicopathological and molecular profiles associated with programmed death-ligand 1 (PD-L1) expression in precancerous lesions and invasive adenocarcinoma in subcentimeter pulmonary nodules.
Patients and Methods
Patients with histologically confirmed atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma (ADC) were included. PD-L1 expression was evaluated at each center using a PD-L1 immunohistochemistry 22C3 pharmDx kit (Agilent, Santa Clara, CA, USA). The tumor proportion score (TPS) cutoff values were set at ≥ 1% and ≥ 50%.
Results
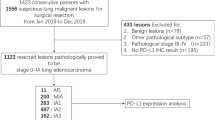
A total of 2022 nodules from 1844 patients were analyzed. Of these, 9 (0.45%) nodules had PD-L1 TPS ≥ 50%, 187 (9.25%) had PD-L1 TPS 1–49%, and 1826 (90.30%) had PD-L1 TPS < 1%. A total of 378 (18.69%), 1016 (50.25%), and 628 (31.06%) nodules were diagnosed as AAH/AIS, MIA, and ADC, respectively, by pathology. A total of 1377 (68.10%), 591 (25.67%), and 54 (2.67%) nodules were diagnosed as pure ground-glass opacity (GGO), mixed GGO, and solid nodules, respectively, by computed tomography. There was a significant difference between PD-L1 expression and anaplastic lymphoma kinase (ALK) mutation status (P < 0.001). PD-L1 expression levels were significantly different from those determined using the International Association for the Study of Lung Cancer (IASLC) grading system (P < 0.001).
Conclusions
PD-L1 expression was significantly associated with radiological and pathological invasiveness and driver mutation status in subcentimeter pulmonary nodules. The significance of PD-L1 expression in the evolution of early-stage lung adenocarcinoma requires further investigation.





Similar content being viewed by others
Availability of Data and Materials
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the tnm stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51.
Oudkerk M, Liu S, Heuvelmans MA, Walter JE, Field JK. Lung cancer LDCT screening and mortality reduction—evidence, pitfalls and future perspectives. Nat Rev Clin Oncol. 2021;18(3):135–51.
Wu F, Tian SP, Jin X, et al. CT and histopathologic characteristics of lung adenocarcinoma with pure ground-glass nodules 10 mm or less in diameter. Eur Radiol. 2017;27(10):4037–43.
Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–63.
Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800.
Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5(9):915–9.
Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71.
Suresh S, Chen B, Zhu J, et al. eIF5B drives integrated stress response-dependent translation of PD-L1 in lung cancer. Nat Cancer. 2020;1(5):533–45.
Carlisle JW, Steuer CE, Owonikoko TK, Saba NF. An update on the immune landscape in lung and head and neck cancers. CA Cancer J Clin. 2020;70(6):505–17.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35.
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65.
Simundza I, Krnic D, Juricic J, et al. Expression of PD-L1 is associated with inflammatory microenvironment in surgical specimens of non-small cell lung cancer. J Pers Med. 2021;11(8):767.
Handa Y, Tsutani Y, Shiroma N, et al. Prognostic impact of programmed death-ligand 1 and surrounding immune status on stage I lung cancer. Clin Lung Cancer. 2020;21(4):e302–14.
Dietel M, Savelov N, Salanova R, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: the global, multicenter EXPRESS study. Lung Cancer. 2019;134:174–9.
Li D, Zhu X, Wang H, Li N. Association between PD-L1 expression and driven gene status in NSCLC: a meta-analysis. Eur J Surg Oncol. 2017;43(7):1372–9.
Lamberti G, Spurr LF, Li Y, et al. Clinicopathological and genomic correlates of programmed cell death ligand 1 (PD-L1) expression in nonsquamous non-small-cell lung cancer. Ann Oncol. 2020;31(6):807–14.
Li CC, Liu J, Xie ZH, et al. PD-L1 expression with respect to driver mutations in non-small cell lung cancer in an Asian population: a large study of 1370 cases in China. Ther Adv Med Oncol. 2020;12:10.
Miyazawa T, Marushima H, Saji H, et al. PD-L1 expression in non-small-cell lung cancer including various adenocarcinoma subtypes. Ann Thorac Cardiovasc Surg. 2019;25(1):1–9.
Zhang C, Zhang J, Xu FP, et al. Genomic landscape and immune microenvironment features of preinvasive and early invasive lung adenocarcinoma. J Thorac Oncol. 2019;14(11):1912–23.
Zhou J, Lin H, Ni Z, et al. Expression of PD-L1 through evolution phase from pre-invasive to invasive lung adenocarcinoma. BMC Pulm Med. 2023;23(1):18.
WHO Classification of Tumours, 5th Edition, Volume 5. 2021; https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Thoracic-Tumours-2021. Accessed 03 July 2021.
Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Torlakovic E, Lim HJ, Adam J, et al. “Interchangeability” of PD-L1 immunohistochemistry assays: a meta-analysis of diagnostic accuracy. Mod Pathol. 2020;33(1):4–17.
Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13(9):1302–11.
Ilie M, Juco J, Huang L, Hofman V, Khambata-Ford S, Hofman P. Use of the 22C3 anti-programmed death-ligand 1 antibody to determine programmed death-ligand 1 expression in cytology samples obtained from non-small cell lung cancer patients. Cancer Cytopathol. 2018;126(4):264–74.
Doroshow DB, Bhalla S, Beasley MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18(6):345–62.
Russell-Goldman E, Kravets S, Dahlberg SE, Sholl LM, Vivero M. Cytologic-histologic correlation of programmed death-ligand 1 immunohistochemistry in lung carcinomas. Cancer Cytopathol. 2018;126(4):253–63.
Skov BG, Skov T. Paired Comparison of PD-L1 expression on cytologic and histologic specimens from malignancies in the lung assessed with PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol. 2017;25(7):453–9.
Sakakibara R, Inamura K, Tambo Y, et al. EBUS-TBNA as a promising method for the evaluation of tumor PD-L1 expression in lung cancer. Clin Lung Cancer. 2017;18(5):527-534.e521.
Organization WH. WHO Classification of tumours of the lung, plura, thymus and heart. 2014.
Moreira AL, Ocampo PSS, Xia Y, et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol. 2020;15(10):1599–610.
Zheng Q, Huang Y, Zeng X, et al. Clinicopathological and molecular characteristics associated with PD-L1 expression in non-small cell lung cancer: a large-scale, multi-center, real-world study in China. J Cancer Res Clin Oncol. 2021;147(5):1547–56.
Li L, Sun RF, Miao Y, et al. PD-1/PD-L1 expression and interaction by automated quantitative immunofluorescent analysis show adverse prognostic impact in patients with diffuse large B-cell lymphoma having T-cell infiltration: a study from the International DLBCL Consortium Program. Mod Pathol. 2019;32(6):741–54.
Toyokawa G, Takada K, Okamoto T, et al. Relevance between programmed death ligand 1 and radiologic invasiveness in pathologic stage I lung adenocarcinoma. Ann Thorac Surg. 2017;103(6):1750–7.
Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–86.
Chen Q, Fu YY, Yue QN, et al. Distribution of PD-L1 expression and its relationship with clinicopathological variables: an audit from 1071 cases of surgically resected non-small cell lung cancer. Int J Clin Exp Pathol. 2019;12(3):774–86.
Naso JR, Wang G, Pender A, et al. Intratumoral heterogeneity in programmed death-ligand 1 immunoreactivity is associated with variation in non-small cell lung carcinoma histotype. Histopathology. 2020;76(3):394–403.
Pan Y, Zheng D, Li Y, et al. Unique distribution of programmed death ligand 1 (PD-L1) expression in East Asian non-small cell lung cancer. J Thorac Dis. 2017;9(8):2579–86.
Huynh TG, Morales-Oyarvide V, Campo MJ, et al. Programmed cell death ligand 1 expression in resected lung adenocarcinomas: association with immune microenvironment. J Thorac Oncol. 2016;11(11):1869–78.
Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thoracic Oncol. 2017;12(2):208–22.
Acknowledgements
We thank our colleagues in the departments of radiology and pathology for their detailed diagnostic reports. We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by the National Natural Science Foundation of China (81872510); Guangdong Provincial People’s Hospital Young Talent Project (GDPPHYTP201902); High-level Hospital Construction Project (DFJH201801); GDPH Scientific Research Funds for Leading Medical Talents and Distinguished Young Scholars in Guangdong Province (No. KJ012019449); Guangdong Basic and Applied Basic Research Foundation (No. 2019B1515130002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
None of the authors have a financial interest or other potential conflict of interest related to subject matter or materials mentioned in the manuscript. Neither any author nor the study received any corporate funding.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Xiao, Y., Hu, H. et al. Expression Changes in Programmed Death Ligand 1 from Precancerous Lesions to Invasive Adenocarcinoma in Subcentimeter Pulmonary Nodules: A Large Study of 2022 Cases in China. Ann Surg Oncol 30, 7400–7411 (2023). https://doi.org/10.1245/s10434-023-14009-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14009-x