Abstract
Background
Intrahepatic cholangiocarcinoma (ICC) is associated with poor long-term outcomes, and limited evidence exists on optimal resection margin width. This study used artificial intelligence to investigate long-term outcomes and optimal margin width in hepatectomy for ICC.
Methods
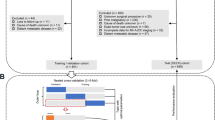
The study enrolled patients who underwent curative-intent resection for ICC between 1990 and 2020. The optimal survival tree (OST) was used to investigate overall (OS) and recurrence-free survival (RFS). An optimal policy tree (OPT) assigned treatment recommendations based on random forest (RF) counterfactual survival probabilities associated with each possible margin width between 0 and 20 mm.
Results
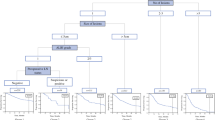
Among 600 patients, the median resection margin was 4 mm (interquartile range [IQR], 2–10). Overall, 379 (63.2 %) patients experienced recurrence with a 5-year RFS of 28.3 % and a 5-year OS of 38.7 %. The OST identified five subgroups of patients with different OS rates based on tumor size, a carbohydrate antigen 19-9 [CA19-9] level higher than 200 U/mL, nodal status, margin width, and age (area under the curve [AUC]: training, 0.81; testing, 0.69). The patients with tumors smaller than 4.8 cm and a margin width of 2.5 mm or greater had a relative increase in 5-year OS of 37 % compared with the entire cohort. The OST for RFS estimated a 46 % improvement in the 5-year RFS for the patients younger than 60 years who had small (<4.8 cm) well- or moderately differentiated tumors without microvascular invasion. The OPT suggested five optimal margin widths to maximize the 5-year OS for the subgroups of patients based on age, tumor size, extent of hepatectomy, and CA19-9 levels.
Conclusions
Artificial intelligence OST identified subgroups within ICC relative to long-term outcomes. Although tumor biology dictated prognosis, the OPT suggested that different margin widths based on patient and disease characteristics may optimize ICC long-term survival.



Similar content being viewed by others
References
Huang T, Liu H, Lin Z, et al. Preoperative prediction of intrahepatic cholangiocarcinoma lymph node metastasis by means of machine learning: a multicenter study in China. BMC Cancer. 2022;22:931.
Nathan H, Pawlik TM, Wolfgang CL, et al. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg. 2007;11:1488–96; discussion 1496–7.
Tan JC, Coburn NG, Baxter NN, et al. Surgical management of intrahepatic cholangiocarcinoma: a population-based study. Ann Surg Oncol. 2008;15:600–8.
The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg. 1989;19:98–129.
Njei B. Changing pattern of epidemiology in intrahepatic cholangiocarcinoma. Hepatology. 2014;60:1107–8.
Saha SK, Zhu AX, Fuchs CS, et al. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21:594–9.
Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149:565–74.
Alaimo L, Moazzam Z, Brown ZJ, et al. Application of hazard function to investigate recurrence of intrahepatic cholangiocarcinoma after curative-intent liver resection: a novel approach to characterize recurrence. Ann Surg Oncol. 2023;30(3):1340–9.
Yamamoto M, Ariizumi S. Intrahepatic recurrence after surgery in patients with intrahepatic cholangiocarcinoma. J Gastroenterol. 2006;41:925–6.
Farges O, Fuks D. Clinical presentation and management of intrahepatic cholangiocarcinoma. Gastroenterol Clin Biol. 2010;34:191–9.
Spolverato G, Kim Y, Ejaz A, et al. Conditional probability of long-term survival after liver resection for intrahepatic cholangiocarcinoma: a multi-institutional analysis of 535 patients. JAMA Surg. 2015;150:538–45.
Loftus TJ, Tighe PJ, Filiberto AC, et al. Artificial intelligence and surgical decision-making. JAMA Surg. 2020;155:148–58.
Bertsimas D, Dunn J, Gibson E, et al. Optimal survival trees. Mach Learn. 2022;111(8):2951–3023.
Amram M, Dunn J, Zhuo YD. Optimal policy trees. Mach Learn. 2022;111:2741–68.
Bertsimas D, Margonis GA, Sujichantararat S, et al. Using artificial intelligence to find the optimal margin width in hepatectomy for colorectal cancer liver metastases. JAMA Surg. 2022;157:e221819.
Shaikh CF, Alaimo L, Moazzam Z, et al. Predicting overall and recurrence-free survival in patients with intrahepatic cholangiocarcinoma using the MEGNA score: a multi-institutional analysis. J Surg Oncol. 2023;127(1):73–80.
Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2:333–39. HPB Oxford. 2002;4:99; author reply 99–100.
Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual. New York: Springer; 2017.
Interpretable AI, LLC. Interpretable AI Documentation. 2023. https://www.interpretable.ai.
Watanabe Y, Matsuyama Y, Izumi N, et al. Effect of surgical margin width after R0 resection for intrahepatic cholangiocarcinoma: a nationwide survey of the Liver Cancer Study Group of Japan. Surgery. 2020;167:793–802.
Spolverato G, Yakoob MY, Kim Y, et al. The impact of surgical margin status on long-term outcome after resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2015;22:4020–8.
Liu H, Lin L, Lin Z, et al. Impact of surgical margin width on long-term outcomes for intrahepatic cholangiocarcinoma: a multicenter study. BMC Cancer. 2021;21:840.
Ma KW, Cheung TT, She WH, et al. The effect of wide resection margin in patients with intrahepatic cholangiocarcinoma: a single-center experience. Medicine Baltimore. 2016;95:e4133.
Bartsch F, Baumgart J, Hoppe-Lotichius M, et al. Intrahepatic cholangiocarcinoma: influence of resection margin and tumor distance to the liver capsule on survival. BMC Surg. 2020;20:61.
Moris D, Palta M, Kim C, et al. Advances in the treatment of intrahepatic cholangiocarcinoma: an overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. 2023;73(2):198–222.
Tsilimigras DI, Hyer JM, Paredes AZ, et al. A novel classification of intrahepatic cholangiocarcinoma phenotypes using machine learning techniques: an international multi-institutional analysis. Ann Surg Oncol. 2020;27:5224–32.
Conci S, Ruzzenente A, Viganò L, et al. Patterns of distribution of hepatic nodules (single, satellites, or multifocal) in intrahepatic cholangiocarcinoma: prognostic impact after surgery. Ann Surg Oncol. 2018;25:3719–27.
Ruzzenente A, Fassan M, Conci S, et al. Cholangiocarcinoma heterogeneity revealed by multigene mutational profiling: clinical and prognostic relevance in surgically resected patients. Ann Surg Oncol. 2016;23:1699–707.
Wei T, Zhang XF, He J, et al. Prognostic impact of perineural invasion in intrahepatic cholangiocarcinoma: multicentre study. Br J Surg. 2022;109:610–6.
Cady B. Basic principles in surgical oncology. Arch Surg. 1997;132:338–46.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alaimo, L., Moazzam, Z., Endo, Y. et al. The Application of Artificial Intelligence to Investigate Long-Term Outcomes and Assess Optimal Margin Width in Hepatectomy for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 30, 4292–4301 (2023). https://doi.org/10.1245/s10434-023-13349-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13349-y