Abstract
Background
The lipid scavenger receptor cluster of differentiation 36 (CD36) has been shown to have a pro-metastatic function in several cancers. Adipose tissue, a favorable site for peritoneal metastasis (PM) from gastric cancer (GC), promotes this process by providing free fatty acids (FFAs); however, the role of CD36 in PM progression from GC remains to be elucidated.
Materials and Methods
We evaluated CD36 expression in the GC cells under various conditions. CD36 overexpressing (CD36OE) MKN45 cells were prepared and their migration and invasive properties were assessed. A PM mouse model was used to investigate the biological effects of palmitic acid (PA) and CD36. Furthermore, we examined the clinical role of CD36 expression in 82 human PM samples by immunohistochemical staining.
Results
Hypoxia markedly increased CD36 expression in GC cells. In normoxia, only CD36OE MKN45 cells treated with PA showed an increase in migration and invasion abilities. An increased expression of active Rac1 and Cdc42 was observed, which decreased following etomoxir treatment. Conversely, hypoxia increased those capacities of both vector and CD36OE MKN45 cells. In a mouse model transplanted with CD36OE MKN45 cells, more peritoneal tumors were observed in the high-fat diet group than those in the normal diet group. In clinical samples, 80% of PM lesions expressed CD36, consistent with hypoxic regions, indicating a significant association with prognosis.
Conclusion
Our findings indicate that a hypoxia in the peritoneal cavity induces CD36 expression in GC cells, which contributes to PM through the uptake of FFAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Peritoneal metastasis (PM) is the most common cause of cancer recurrence and distant metastasis in gastric cancer (GC).1 The metastatic process occurs through a multistep cascade that is closely related to the molecular alterations present in both the cancer cells and the tumor microenvironment (TME).2,3 PM from GC preferentially occurs in adipocyte-rich organs such as the omentum, where adipocytes are the primary cellular components of the PM microenvironment. Emerging evidence indicates that adipocytes adjacent to invasive cancer cells, referred to as cancer-associated adipocytes, provide free fatty acids (FFAs), growth factors, and cytokines, and transdifferentiate into other stromal cells to alter tumor growth, metastasis, and drug responses.4,5 We also reported that adipocytes dedifferentiated into cancer-associated fibroblast-like immature cells upon contact with GC cells and increased the expression of IL-6 and plasminogen activator inhibitor-1, thereby enhancing the malignant potential of GC cells.6 However, molecular alterations in cancer cells induced by their contact with adipose tissue have not been completely elucidated.
The dominant energy-producing mechanism in cancer cells is glycolysis, which is known as the Warburg effect. However, tumor cells undergoing extracellular matrix detachment have decreased glucose uptake and lower glycolytic activity.7 Recently, fatty acid oxidation (FAO) was found to be the preferred alternative mechanism of energy production, which was dependent on mitochondrial oxidative phosphorylation in anoikis-resistant cells, such as circulating tumor cells and tumor-forming cells.8,9 In particular, the expression of cluster of differentiation 36 (CD36), a scavenger receptor that functions in the high-affinity uptake of long-chain fatty acids, was reported to be involved in the initiation of metastasis via FAO in oral squamous cell carcinoma.10 Accumulating evidence has confirmed that CD36 allows cells to take up lipids from the extracellular microenvironment and promotes FAO to produce ATP,11,12 potentially energizing tumor progression and metastasis.13,14,15,16
Resistance to anoikis is a hallmark of PM because intraperitoneal free cancer cells that have migrated from the primary tumor survive in an anchorage-independent manner.17 Therefore, GC cells require sufficient ATP generation, increased biosynthesis of biomolecules, and maintenance of an appropriate redox status despite the low oxygen (hypoxia) and nutrient levels within the peritoneal cavity. Little is known about the expression of CD36 and its mechanistic role in PM. Therefore, we hypothesized that CD36-dependent uptake and FAO using adipocyte-derived FFAs may be involved in the PM process.
In the present study, we used hypoxic conditions to assess the CD36 expression in GC cells and their contribution to the development of PM in vitro and in vivo. Furthermore, we also analyzed the CD36 expression in PM human samples using immunohistochemistry.
Materials and Methods
Ethical Approval
This study was approved by the Institutional Review Board of Kanazawa University Graduate School of Medical Science. Written informed consent was obtained from each patient. The present study was conducted in accordance with the ethical standards laid out by the institutional and national committees on human experimentation and the Helsinki Declaration of 1964 and later versions.
Cells and Materials
GC cell lines MKN45, NUGC-4, and MKN74 were purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). The cells were exposed to hypoxia (0.1% O2) using a BIONIX system (Sugiyama-gen Co.). Palmitic acid (PA; P9767, Sigma Aldrich, UK) was prepared as 2 mM stock solution by dissolving in 75% ethanol at 70 °C. Stock solutions were then added to 2% FA-free bovine serum albumin (BSA; 017-15146, FUJIFILM, Japan) to achieve the desired final FA concentration. PA or etomoxir (10 mM in dimethyl sulfoxide [DMSO], 124083-20-1, ChemScene, Japan) was added to the medium, and 75% ethanol and DMSO were used as respective controls.
Immunofluorescence Staining
Immunofluorescence was performed as described in a previous study.18 Briefly, slides with the samples were incubated with CD36 antibody (NB400-144, rabbit, diluted 1:300, Novus Biologicals, Littleton, CO, USA) overnight at 4 °C. Immunoreactivity was visualized using anti-rabbit immunoglobulin (Ig) G secondary antibody conjugated with Alexa Fluor® 546 (1:400; Molecular Probes/Invitrogen, Eugene, OR, USA). The cells were then incubated with DAPI for nuclear staining. The slides were observed using an immunofluorescence microscope (BX50/BS-FLA; Olympus, Tokyo, Japan).
Flow Cytometry
Cells were incubated for 1 h with fluorescein isothiocyanate (FITC) anti-human CD36 antibody (336203; BioLegend, San Diego, CA, USA) at a 5:100 dilution in 100 μL of flow cytometry staining (FACS) buffer (2 mL fetal bovine serum (FBS)/100 mL phosphate-buffered saline [PBS]). Subsequently, the cells were washed twice with FACS buffer, followed by centrifugation at 1500 rpm for 3 min. Cell pellet was resuspended in 0.4 mL of FACS buffer and acquired on an Attune acoustic cytometer (Applied Biosystems, Life Technologies, Carlsbad, CA, USA). Analysis was performed using FlowJo v 9.6.1 software (FlowJo, Ashland, OR, USA).
Cluster of Differentiation 36 (CD36) Plasmid Construction and Transfection
Complementary DNA (cDNA) was prepared and purified from messenger RNA (mRNA) extracted from human monocytes using a reverse transcription reaction (primers: 5′-GAGAATTCTTAACACTAATTCACCTCCT-3′ and 5′-GATCTAGATTATTTTATTGTTTTCGATC-3′) and amplified by polymerase chain reaction (PCR). After restriction digestion (EcoRI and XbaI; Takara Bio, Kusatsu, Shiga, Japan) of cDNA and vector (pCI-neo mammalian expression vector, E1841; Promega Corporation, Madison, WI, USA), the cDNA was ligated with the vector. cDNA expression vector was then transfected into MKN45 cells using Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Cells were cultured in the presence of G418 (400 μg/mL) post-transfection, and the G418-resistant colonies were picked after 2 weeks. Stably transfected cells were seeded in 96-well plates at a density of two cells/well for clonal expansion, and were gradually scaled up from 96 wells to 6 wells. Among the clonally expanded colonies, the groups with the highest expression of CD36 were selected. CD36 expression was verified using Western blotting and flow cytometry.
Transwell Migration, Invasion Assays
Transwell migration and invasion assays were performed using 8 μm Transwell inserts (Migration Chamber: 140629, Lab Unlimited, Dublin, Ireland; Invasion Chamber: 354480, Corning Inc., Corning, NY, USA). Cells were suspended in serum-free RPMI-1640 medium without glucose or glutamine, containing 2% BSA and cultured in the upper chamber. 1% FBS-conditioned serum-free, no glucose, no glutamine RPMI-1640 with 2% BSA containing either 0.1 mM PA, 0.1 mM PA with 50 or 100 μM etomoxir, or respective controls, was added to the lower chamber. The cells that migrated or invaded the lower chamber of the inserts were photographed under a microscope (BX50/BS-FLA; Olympus), and five visual fields were randomly chosen to calculate the number of cells.
Western Blotting
Western blotting was performed according to a previously reported standard protocol.18 The CD36 SR-B3 antibody (NB400-144, rabbit polyclonal IgG, diluted 1:1000, Novus Biologicals) was used. Rac1 and Cdc42 levels were measured using an Rac1/Cdc42 Activation Assay Kit (Sigma-Aldrich, Gillingham, Dorset, UK) according to the manufacturer's protocols. CD36- or vector-transfected MKN45 cells were incubated with etomoxir (100 μM) or 1% DMSO for 4 h, followed by exposure to PA (0.1 mM) or 3.75% ethanol in normoxia.
Mouse Peritoneal Metastatic Model
All animal experiments were performed in accordance with Kanazawa University standard guidelines. Female immunocompromised BALB/c-nu/nu mice aged 4–6 weeks were maintained in a sterile environment. We randomly divided 20 mice into four groups and created GC peritoneal metastatic models using CD36 overexpressing (CD36OE) MKN45 cells or empty vector. Two groups of mice were fed a 60 kcal% high-fat diet (HFD; Research Diets, New Brunswick, NJ, USA) and the other two groups were fed a normal diet (ND). After 1 week of feeding, either 1 × 107 vector or CD36OE MKN45 cells were intraperitoneally injected into mice in each group. All mice were sacrificed by cervical dislocation method 2 weeks after injection, and the size and number of peritoneal nodules. Plasma triglycerides were measured using the Mouse Triglyceride ELISA Kit (My BioSource, San Diego, CA, USA) according to the manufacturer’s protocols.
Patient Population
Eighty-two patients who underwent biopsy or resection of PM of GC from 2000 to 2016 were selected as the study subjects. Of these, 67 were GC patients with PM at the time of initial presentation, and 15 were patients of peritoneal recurrence after R0 gastrectomy. Of the former, 44 patients underwent gastrectomy, 18 of whom had combined resections of organs such as small and large intestines due to the invasion of PM. In five of the peritoneal recurrence cases, bowel resection was performed for intestinal stenosis. Fifty-nine patients underwent biopsy or local excision of PM for diagnosis or macroscopic curative surgery, and 78 patients received chemotherapy, of whom 62 patients received systemic chemotherapy plus intraperitoneal chemotherapy. We examined the expression of CD36 and carbonic anhydrase IX (CAIX) in the primary and PM lesion samples of GC. We also evaluated 36 lymph node metastatic lesions in patients who underwent combined resection of PM and primary lesions. In addition, liver metastatic lesions were evaluated in 19 different patients.
Immunohistochemical Staining
Immunohistochemical (IHC) staining was performed according to a previously reported standard protocol.18 CD36 antibody (NB400-144, rabbit, diluted 1:300; Novus Biologicals) and CAIX antibody (NB100-417, rabbit, diluted 1:500; Novus Biologicals) were used for the IHC assessment. Based on intensity, the staining was graded/scored as no staining (0), weak staining (1), moderate staining (2), and strong staining (3). The number of positively stained GC cells was divided into the following four ranges (proportion score): ≤5% (0), 6–25% (1), 26–50% (2), and >51% (3). The final staining score was calculated using the following formula: overall score = intensity score × proportion score. A final score of ≤4 was defined as negative staining and >4 as positive staining.
Statistical Analysis
Statistical analyses were conducted using EZR statistical software version 1.32. We adopted the upper limit at our hospital as the cut-off value for C-reactive protein (CRP), carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, and CA125. For prognostic nutritional index, platelet, and body mass index (BMI), a receiver operating characteristic curve was created based on the median survival time, and the nearest point in the upper left corner was set as the best value. The Chi-square test was used to determine the differences in CD36 expression and clinicopathological parameters, and the Kaplan–Meier method and log-rank test were used for survival analysis. Overall survival (OS) was calculated based on the date of diagnosis of PM or peritoneal recurrence. Univariate and multivariate analyses were performed using the Cox hazard model as a prognostic parameter. Statistical significance was set at p < 0.05.
Results
Hypoxia Increased CD36 Expression in Gastric Cancer (GC) Cells
Basal CD36 expression in MKN45, NUGC-4, and MKN74 cells was confirmed using flow cytometry and was found to be low, ranging from 0.5 to 3% (Fig. 1a). Similarly, fluorescence immunostaining revealed a fewer number of CD36-positive GC cells (Fig. 1b). Furthermore, CD36 expression was assessed in MKN45 cells under hypoxic conditions with no glucose, no glutamate media, and palmitate administration (Fig. 2). Under hypoxia, CD36 expression increased to 20.1% and further increased to 60.6% with no glucose, no glutamine, and the addition of PA. In contrast, no glucose, glutamine, or addition of PA increased the CD36 expression in MKN45 cells marginally under normoxia (Fig. 1c).
CD36 expression in gastric cancer cell lines. a Flow cytometry analysis showing the expression of CD36 in MKN45, NUGC-4, and MKN74 cells. b Immunofluorescence staining showing the expression of CD36 in MKN45, NUGC-4, and MKN74 cells. c Hypoxia increased CD36 expression in MKN45 cells. Flow cytometry analysis of CD36 expression in MKN45 cells after 24 h of incubation under conditions of hypoxia, no glucose, no glutamate, and palmitic acid administration
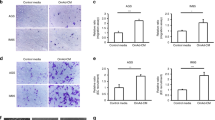
a Flow cytometric analysis of the transfection efficiencies of control vector or CD36 overexpression (CD36OE) MKN45 cells. b Western blot analysis of the transfection efficiencies of control vector or CD36OE MKN45 cells. c Transwell migration and invasion assay of vector and CD36 OE MKN45 cells after 0.1 mM PA/50 μM or 100 μM etomoxir treatment in normoxia. d Transwell migration and invasion assay of vector and CD36 OE MKN45 cells after 0.1 mM PA/50 μM or 100 μM etomoxir treatment in hypoxia. e Rac1 and Cdc42 were measured using an Rac1/Cdc42 Activation Assay Kit. Vector and CD36OE MKN45 cells were subjected to 16 h fatty acid-free starvation and were then treated with etomoxir (100 μM) or 1% DMSO for 4 h, followed by exposure with PA (0.1 mM) or 3.75% ethanol in normoxia. PA palmitic acid
Addition of Palmitic Acid Promoted GC Cell Migration and Invasion Through CD36 in Both Normoxia and Hypoxia
We aimed to assess whether exogenous PA promoted the migration and invasion of GC cells mediated by CD36. To mimic the heterogeneity and instability of CD36 expression in GC cells, we transfected the GC cell line MKN45 with CD36 expression plasmid and its control. Stably transfected cells showing similar CD36 expression levels, as analyzed by flow cytometry and Western blotting, were expanded from a large pool of samples and used for subsequent experiments (Figs. 2a, b).
We evaluated the migration and invasion properties of CD36OE MKN45 cells. Cells were divided into four groups according to the presence or absence of PA in the lower chamber and the presence or absence of etomoxir, a CPT1 inhibitor, in the upper chamber. Under normoxia, CD36OE cells treated with 0.1 mM PA showed increased migration and invasion capacity, which was inhibited by 100 µM etomoxir. Conversely, in the control vector cells, neither migration nor invasion capacity were altered by PA or etomoxir treatment (Fig. 2c).
Under hypoxia, the migration and invasive capacity of both the control vector as well as CD36OE MKN45 cells significantly increased with the addition of 0.1 mM PA. Furthermore, treatment with 50 and 100 μM etomoxir suppressed the enhanced migration and invasive ability observed with PA treatment in both the control vector and CD36OE MKN45 cells (Fig. 2d).
Rho GTPases, such as Rac1 and Cdc42, are well known for their roles in regulating cell migration and invasion. In CD36OE MKN45 cells, active Rac1 and Cdc42 were found to increase with PA stimulation, but were decreased post-etomoxir treatment (Fig. 2e). No changes were observed in the control vector cells under similar conditions (Fig. 2e). These findings suggested that the increase in active Rac1 and Cdc42 possibly mediates the enhanced migratory and invasive capacity in CD36OE cells compared with that in the control vector cells.
High-Fat Diet Promoted the Growth of Peritoneally Implanted CD36 Overexpressing MKN45 Cells in Mice
To determine the differences in the dissemination ability induced by CD36-mediated FFA changes, control vector or CD36OE MKN45 cells were intraperitoneally injected into nude mice. An ND or HFD was then administered to the mouse model and their ability to form peritoneal tumors was compared. There were no significant differences in the body weights of mice in each group during the observation period (data not shown). The plasma triglyceride levels were higher in mice fed an HFD than those in mice fed an ND (Fig. 3a), indicating that FFAs promoted the growth of peritoneally implanted GC cells.
HFDs promoted the growth of peritoneally implanted CD36 overexpression (CD36OE) MKN45 cells in mice. a Plasma triglyceride levels in nude mice fed an ND or HFD. b Representative macroscopic images of peritoneal tumors (arrows and circles) in four groups of mouse models inoculated intraperitoneally with vector or CD36 OE MKN45 cells and fed an ND or HFD. The results of the number of peritoneal tumors and tumor weight are expressed as the mean ± SEM (n = 5, *p < 0.05). c Immunohistochemical staining of CD36 expression in peritoneal tumors of vector or CD36 OE MKN45 cells. HFD high-fat diet, ND normal diet, SEM standard error of the mean
There were fewer peritoneal nodules in the vector cells/ND group compared with those in the vector cells/HFD group, however this difference was not significant (Fig. 3b). In mice with CD36OE MKN45 cell transplants, the HFD group had significantly more peritoneal tumor nodules and tumor weight than that in the ND group. Histological analysis of the xenografts also confirmed high CD36 expression in mice with CD36OE cell transplants (Fig. 3c).
CD36 Expression in Clinical GC Tissue Samples
CD36 expression increased in PM samples compared with their primary tumor counterparts (p < 0.05). In addition, CD36 expression in lymph node and liver metastatic tissues was significantly lower than that in PM tissue. The frequency of CD36 expression in the corresponding primary lesions, PM lesions, and lymph node lesions in the same cases was also comparable (Fig. 4a).
a Representative microscopic images of CD36 expression in primary tumors, and peritoneal, liver and lymph node metastases. Staining intensity of CD36 expression was calculated and the frequency of high expression in each organ was compared using the Chi-square test (*p < 0.05). b Representative microscopic images of CAIX expression in primary tumors, and peritoneal, liver and lymph node metastases. Staining intensity of CAIX expression was calculated and the frequency of high expression in each organ was compared using the Chi-square test (*p < 0.05). c Co-localization of CD36 and CAIX expression was evaluated in serial sections. CD36 expression is associated with CAIX expression (*p < 0.05)
Since hypoxia modulates CD36 expression in vitro, we evaluated intratumoral hypoxia in primary tumors and metastatic tissues using CAIX staining as an endogenous surrogate marker of hypoxia. The CAIX staining index of peritoneal metastatic tissues was higher than that of the primary tumor, lymph node metastasis, and liver metastatic tissues. The expression of CAIX in the corresponding PM, primary tumor, and lymph node lesions in the same cases also showed similar results (Fig. 4b). Furthermore, we confirmed that CD36 expression was associated with CAIX expression using serial sections of the PM (Fig. 4c).
High CD36 Expression and Overall Survival of GC Patients with Peritoneal Metastasis
We analyzed the relationship between CD36 expression and clinicopathological factors in GC patients with PM (Table 1), and found there was no significant difference in CD36 expression among histological differentiation in primary tumors and PM. Moreover, there was no difference in CD36 expression by tumor size between peritoneal specimens with combined bowel resection and those resected by biopsy or local excision. In addition, CD36 expression according to the location of resected PM lesions was examined, but there was no difference in CD36 expression between parietal and visceral peritoneal specimens.
As shown in Fig. 5, OS was significantly lower in PM patients with high CD36 expression than in those with low CD36 expression (p = 0.026).
To assess the prognostic potential of clinicopathological factors, including CD36, in GC patients with PM, univariate and multivariate analyses were performed using a Cox proportional hazards model (Table 2). In the univariate analysis, significant differences were found in chemotherapy, P stage (P1a/P1b, c), CA19-9, CRP, and CD36. Next, we performed multivariate analysis to determine the independent factors affecting OS. Variables with p-values <0.05 in the univariate analysis were included, and chemotherapy and CD36 were extracted as an independent prognostic factor for OS.
Discussion
The importance of CD36 in the regulation of proliferation, metastasis, and angiogenesis has been demonstrated, and its expression in primary tumors has been reported to be correlated with poor prognosis in different types of tumors.19,20,21 In this study, we report that a matched cohort of primary and PM tumors using human clinical samples showed upregulation of CD36 in PM tissue, and CD36 expression correlated with poor prognosis in patients with PM. We observed that CD36 expression was highly upregulated in hypoxia under glucose and glutamine deprivation and the addition of PA. In contrast, CD36 expression in response to these stimuli did not change under normoxia, suggesting that hypoxia is the primary driving force that induces CD36 expression in GC cells. As demonstrated by Kastelein et al. using three-dimensional whole-tumor imaging, hypoxic conditions in PM are due to poor perfusion of the microvasculature.22 Consistent with their findings, our clinical samples also showed more frequent and prominent CAIX-positive regions, reflecting hypoxia in PM relative to the primary tumor and lymphatic and liver metastases, and CD36 expression was upregulated, consistent with CAIX expression. Although tumors generally have an hypoxic environment in the central part as the tumor size increases, immunostaining of clinical specimens showed no difference in CD36 expression depending on the tumor size of the PM. We believe this is because most PM lesions, regardless of tumor size, have an hypoxic environment due to high intratumor pressure caused by abundant fibrotic stroma, which obstructs blood perfusion.23 In terms of the adipocyte environment, there was no difference in CD36 expression between the lesions on the adipocyte-rich visceral peritoneum, such as greater omentum or mesentery, and those on the adipocyte-poor parietal peritoneum. Comparisons based on BMI also showed no significant differences in CD36 expression. Thus, these results indicate hypoxia in TME was the most important factor affecting CD36 expression.
Under normoxia, CD36OE GC cells displayed enhanced migratory and invasive capacity and increased expression of active Rac1 and Cdc42 following PA treatment, which was suppressed by etomoxir. In hypoxia, similar changes were observed in control vector cells after PA treatment and etomoxir administration, although CD36OE cells showed a more prominent response. Our findings suggest that hypoxia in the peritoneal cavity enhances CD36 expression in GC cells and promotes the migratory and invasive abilities of cancer cells via the intracellular uptake of adipocyte-derived exogenous PA. Although HFD administration significantly increased peritoneal tumors in mouse models transplanted with CD36OE cells, the adipocyte environment, such as PM location and BMI, did not correlate with survival in human data. This discrepancy may be due to the extremely low visceral fat content of nude mice compared with humans. We suggest that in humans, FFA is supplied by adipocytes in the abdominal cavity, whereas in nude mice, FFA is supplied to CD36OE cells from systemic circulation as a result of an HFD.
Enhanced CD36 expression has been reported in hypoxic environments in non-malignant diseases. In hepatocytes, an hypoxic environment, as represented by obstructive sleep apnea, enhances CD36 expression on the plasma membrane and contributes to the onset of non-alcoholic fatty liver disease by increasing FFA uptake.24 In addition, hypoxia-inducible factor (HIF)-1α, which is considered the master regulator of oxygen homeostasis, upregulates CD36 expression and function in retinal epithelial cells and macrophages.25,26 Although hypoxia is considered a major stimulus for numerous malignancies through angiogenesis and tumor stemness, the molecular mechanisms governing the regulation of CD36 by hypoxia in malignant tumors are yet to be elucidated. Therefore, one of the most surprising findings of our study was that hypoxia induces CD36 expression in GC cells.
Although this study did not analyze the intracellular lipid metabolism of FFAs, several studies have reported increased lipid metabolism during hypoxia.27,28 Bensaad et al. reported that transcriptional regulation of fatty acid-binding proteins (FABP) 3 and FABP7 in glioma and breast cancer cells is induced by HIF-1α and leads to a significant lipid droplet (LD) accumulation during hypoxia.29 They demonstrated that triglycerides contained in LDs are degraded to generate FFAs when the cells need to produce ATP via FAO. FAO was recently found to be essential for the development of anoikis resistance and metastatic phenotypes in various cancers.10,30,31,32 Resistance to anoikis is thought to be a hallmark of PM as cells survive in a free state in the peritoneal cavity. Our study predicts that CD36-mediated FAO using FFAs derived from the peritoneal environment is involved in the development of PM.
Conclusion
Our study demonstrates that (1) CD36 expression is upregulated in GC cells in response to hypoxia; (2) elevated CD36 expression contributes to the migratory and invasive abilities of GC cells and the peritoneal tumor growth using exogenous FFAs; and (3) PM exhibits high CD36 expression, which correlates with its prognosis. The regulation of CD36 expression by hypoxia may be a critical step in the development and progression of PM from GC. These data provide a valuable framework for future studies investigating the metabolic reprogramming of GC cells in the development of PM, where CD36 may be a promising new therapeutic target for PM.
References
Thomassen I, van Gestel YR, van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134(3):622–8.
Li W, Ng JM, Wong CC, Ng EKW, Yu J. Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer. Oncogene. 2018;37(36):4903–20.
Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. 2016;352(6282):169–75.
Takehara M, Sato Y, Kimura T, et al. Cancer-associated adipocytes promote pancreatic cancer progression through SAA1 expression. Cancer Sci. 2020;111(8):2883–94.
Zhao C, Wu M, Zeng N, et al. Cancer-associated adipocytes: emerging supporters in breast cancer. J Exp Clin Cancer Res. 2020;39(1):156.
Hamabe-Horiike T, Harada S, Yoshida K, et al. Adipocytes contribute to tumor progression and invasion of peritoneal metastasis by interacting with gastric cancer cells as cancer associated fibroblasts. Cancer Rep (Hoboken). 2022. https://doi.org/10.1002/cnr2.1647.
Zhao Y, Hu X, Liu Y, et al. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol cancer. 2017;16(1):79.
Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485(7400):661–5.
LeBleu VS, O’Connell JT, Gonzalez Herrera KN, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10):992–1003.
Pascual G, Avgustinova A, Mejetta S, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541(7635):41–5.
Coburn CT, Knapp FF Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275(42):32523–9.
Pepino MY, Kuda O, Samovski D, Abumrad NA. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr. 2014;34:281–303.
Kuemmerle NB, Rysman E, Lombardo PS, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Therapeutics. 2011;10(3):427–36.
Nath A, Li I, Roberts LR, Chan C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep. 2015;5:14752.
Pan J, Fan Z, Wang Z, et al. CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3β/β-catenin pathway. J Exp Clin Cancer Res. 2019;38(1):52.
Ladanyi A, Mukherjee A, Kenny HA, et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018;37(17):2285–301.
Du S, Yang Z, Lu X, et al. Anoikis resistant gastric cancer cells promote angiogenesis and peritoneal metastasis through C/EBPβ-mediated PDGFB autocrine and paracrine signaling. Oncogene. 2021;40(38):5764–79.
Nakamura Y, Kinoshita J, Yamaguchi T, et al. Crosstalk between cancer-associated fibroblasts and immune cells in peritoneal metastasis: inhibition in the migration of M2 macrophages and mast cells by Tranilast. Gastric Cancer. 2022;25(3):515–26.
Hale JS, Otvos B, Sinyuk M, et al. Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cells (Dayton, Ohio). 2014;32(7):1746–58.
Watt MJ, Clark AK, Selth LA, et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci Trans Med. 2019. https://doi.org/10.1126/scitranslmed.aau5758.
Jiang M, Wu N, Xu B, et al. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics. 2019;9(18):5359–73.
Kastelein AW, Vos LMC, van Baal J, et al. Poor perfusion of the microvasculature in peritoneal metastases of ovarian cancer. Clin Exp Metastasis. 2020;37(2):293–304.
Carr RM, Fernandez-Zapico ME. Pancreatic cancer microenvironment, to target or not to target? EMBO Mol Med. 2016;8(2):80–2.
Rey E, Meléndez-Rodríguez F, Marañón P, et al. Hypoxia-inducible factor 2α drives hepatosteatosis through the fatty acid translocase CD36. Liver Int. 2020;40(10):2553–67.
Crucet M, Wüst SJ, Spielmann P, Lüscher TF, Wenger RH, Matter CM. Hypoxia enhances lipid uptake in macrophages: role of the scavenger receptors Lox1, SRA, and CD36. Atherosclerosis. 2013;229(1):110–7.
Mwaikambo BR, Yang C, Chemtob S, Hardy P. Hypoxia up-regulates CD36 expression and function via hypoxia-inducible factor-1- and phosphatidylinositol 3-kinase-dependent mechanisms. J Biol Chem. 2009;284(39):26695–707.
Laurenti G, Benedetti E, D’Angelo B, et al. Hypoxia induces peroxisome proliferator-activated receptor α (PPARα) and lipid metabolism peroxisomal enzymes in human glioblastoma cells. J Cell Biochem. 2011;112(12):3891–901.
Shen GM, Zhao YZ, Chen MT, et al. Hypoxia-inducible factor-1 (HIF-1) promotes LDL and VLDL uptake through inducing VLDLR under hypoxia. Biochem J. 2012;441(2):675–83.
Bensaad K, Favaro E, Lewis CA, et al. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014;9(1):349–65.
Wright HJ, Hou J, Xu B, et al. CDCP1 drives triple-negative breast cancer metastasis through reduction of lipid-droplet abundance and stimulation of fatty acid oxidation. Proc Natl Acad Sci USA. 2017;114(32):E6556-65.
Lee CK, Jeong SH, Jang C, et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363(6427):644–9.
van Weverwijk A, Koundouros N, Iravani M, et al. Metabolic adaptability in metastatic breast cancer by AKR1B10-dependent balancing of glycolysis and fatty acid oxidation. Nat Commun. 2019;10(1):2698.
Acknowledgment
The authors thank Editage (www.editage.com) for English-language editing.
Funding
This study was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Grant Number 19K09166).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Tatsuya Aoki, Jun Kinoshita, Seiichi Munesue, Toshihide Hamabe-Horiike, Takahisa Yamaguchi, Yusuke Nakamura, Koichi Okamoto, Hideki Moriyama, Keishi Nakamura, Shinichi Harada Yasuhiko Yamamoto, Noriyuki Inaki, and Sachio Fushida declare they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aoki, T., Kinoshita, J., Munesue, S. et al. Hypoxia-Induced CD36 Expression in Gastric Cancer Cells Promotes Peritoneal Metastasis via Fatty Acid Uptake. Ann Surg Oncol 30, 3125–3136 (2023). https://doi.org/10.1245/s10434-022-12465-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12465-5