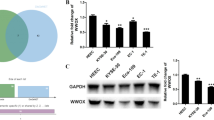
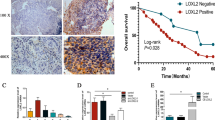
Abstract
Background
NADPH oxidases (NOXs) are transmembrane proteins that generate reactive oxygen species. Recent studies have reported that NOXs are involved in tumor progression in various cancers. However, the expression and role of NOX2 in esophageal squamous cell carcinoma (ESCC) remain unclear. This study aimed to clarify the pathophysiologic role of NOX2 in patients with ESCC and cell lines.
Methods
Two human ESCC cell lines (TE5 and KYSE170) were used for NOX2 transfection experiments, and the effects on cell proliferation, cell cycle, cell motility, and cell survival were analyzed. An mRNA microarray analysis was also performed to assess gene expression profiles. Additionally, NOX2 immunohistochemistry was performed on 130 primary ESCC tumor samples to assess the prognostic value of NOX2 in patients with ESCC.
Results
NOX2 depletion significantly inhibited cell proliferation with the G0/G1 arrest and resulted in apoptosis in two cell lines. Microarray analysis revealed a strong relationship between NOX2 gene expression and the signaling pathway of cell cycle regulation by the B-cell translocation gene 2 (BTG2) family, including BTG2, CCNE2, E2F1, and CDK2 genes. Immunohistochemical staining revealed that high NOX2 protein expression was significantly associated with deeper tumor invasion and selected as one of the independent prognostic factors associated with the 5-year OS rate in patients with ESCC.
Conclusions
NOX2 expression in ESCC cells affects tumorigenesis, especially cell cycle progression via the BTG2-related signaling pathway, as well as the prognosis of patients with ESCC. NOX2 may be a novel biomarker and therapeutic target for ESCC.




Similar content being viewed by others
References
Makhezer N, Ben Khemis M, Liu D, et al. NOX1-derived ROS drive the expression of Lipocalin-2 in colonic epithelial cells in inflammatory conditions. Mucosal Immunol. 2019;12(1):117–31.
Sorce S, Stocker R, Seredenina T, et al. NADPH oxidases as drug targets and biomarkers in neurodegenerative diseases: what is the evidence? Free Radic Biol Med. 2017;112:387–96.
Block K, Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer. 2012;12(9):627–37.
Kamata T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci. 2009;100(8):1382–8.
Mi T, Wang Z, Bunting KD. The cooperative relationship between STAT5 and reactive oxygen species in leukemia: mechanism and therapeutic potential. Cancers (Basel). 2018;10(10):359.
Du S, Miao J, Zhu Z, et al. NADPH oxidase 4 regulates anoikis resistance of gastric cancer cells through the generation of reactive oxygen species and the induction of EGFR. Cell Death Dis. 2018;9(10):948.
Zibara K, Zeidan A, Bjeije H, Kassem N, Badran B, El-Zein N. ROS mediates interferon gamma induced phosphorylation of Src, through the Raf/ERK pathway, in MCF-7 human breast cancer cell line. J Cell Commun Signal. 2017;11(1):57–67.
Gabig TG, Babior BM. The O2(-)-forming oxidase responsible for the respiratory burst in human neutrophils Properties of the solubilized enzyme. J Biol Chem. 1979;254(18):90709–907074.
Grauers Wiktorin H, Aydin E, Hellstrand K, Martner A. NOX2-derived reactive oxygen species in cancer. Oxid Med Cell Longev. 2020;2020:7095902.
Kiffin R, Grauers Wiktorin H, Nilsson MS, et al. Anti-leukemic properties of histamine in monocytic leukemia: the role of NOX2. Front Oncol. 2018;8:218.
Aurelius J, Hallner A, Werlenius O, et al. NOX2-dependent immunosuppression in chronic myelomonocytic leukemia. J Leukoc Biol. 2017;102(2):459–66.
Aydin E, Johansson J, Nazir FH, Hellstrand K, Martner A. Role of NOX2-derived reactive oxygen species in NK Cell-mediated control of murine melanoma metastasis. Cancer Immunol Res. 2017;5(9):804–11.
Martner A, Wiktorin HG, Lenox B, et al. Histamine promotes the development of monocyte-derived dendritic cells and reduces tumor growth by targeting the myeloid NADPH oxidase. J Immunol. 2015;194(10):5014–21.
Harrison IP, Vinh A, Johnson IRD, et al. NOX2 oxidase expressed in endosomes promotes cell proliferation and prostate tumour development. Oncotarget. 2018;9(83):35378–93.
Wang P, Shi Q, Deng WH, et al. Relationship between expression of NADPH oxidase 2 and invasion and prognosis of human gastric cancer. World J Gastroenterol. 2015;21(20):6271–9.
Wang Z, Tang T, Wang S, et al. Aloin inhibits the proliferation and migration of gastric cancer cells by regulating NOX2-ROS-Mediated pro-survival signal pathways. Drug Des Devel Ther. 2020;14:145–55.
Guo Y, Han B, Luo K, Ren Z, Cai L, Sun L. NOX2-ROS-HIF-1α signaling is critical for the inhibitory effect of oleanolic acid on rectal cancer cell proliferation. Biomed Pharmacother. 2017;85:733–9.
Fan Z, Duan X, Cai H, et al. Curcumin inhibits the invasion of lung cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling pathway. Oncol Rep. 2015;34(2):691–8.
Yang WH, Huang Z, Wu J, Ding CC, Murphy SK, Chi JT. A TAZ-ANGPTL4-NOX2 axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancer. Mol Cancer Res. 2020;18(1):79–90.
Liu L, Rezvani HR, Back JH, et al. Inhibition of p38 MAPK signaling augments skin tumorigenesis via NOX2 driven ROS generation. PLoS One. 2014;9(5):e97245.
Lin RJ, Huang Z, Wang SL, et al. Clinicopathological and prognostic value of NADPH oxidase 2 (NOX2) in primary osteosarcoma. J Orthop Sci. 2021;26(3):466–72.
Shimizu H, Shiozaki A, Ichikawa D, et al. The expression and role of Aquaporin 5 in esophageal squamous cell carcinoma. J Gastroenterol. 2014;49(4):655–66.
Brierley JDGM, Wittekind C. TNM Classification of Malignant Tumours (UICC). 8th edn. New York: Wiley-Blackwell; 2017.
Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000;19(1):113–32.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313.
Laurent E, McCoy JW 3rd, Macina RA, et al. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int J Cancer. 2008;123(1):100–7.
Wang HP, Wang X, Gong LF, et al. Nox1 promotes colon cancer cell metastasis via activation of the ADAM17 pathway. Eur Rev Med Pharmacol Sci. 2016;20(21):4474–81.
Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279(33):34643–54.
Luxen S, Belinsky SA, Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 2008;68(4):1037–45.
Pizzolla A, Hultqvist M, Nilson B, et al. Reactive oxygen species produced by the NADPH oxidase 2 complex in monocytes protect mice from bacterial infections. J Immunol. 2012;188(10):5003–11.
Zhong J, Olsson LM, Urbonaviciute V, Yang M, Bäckdahl L, Holmdahl R. Association of NOX2 subunits genetic variants with autoimmune diseases. Free Radic Biol Med. 2018;125:72–80.
Rouault JP, Falette N, Guéhenneux F, et al. Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nat Genet. 1996;14(4):482–6.
Yuniati L, Scheijen B, van der Meer LT, van Leeuwen FN. Tumor suppressors BTG1 and BTG2: beyond growth control. J Cell Physiol. 2019;234(5):5379–89.
Guardavaccaro D, Corrente G, Covone F, et al. Arrest of G(1)-S progression by the p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1 transcription. Mol Cell Biol. 2000;20(5):1797–815.
Lim IK, Lee MS, Ryu MS, et al. Induction of growth inhibition of 293 cells by downregulation of the cyclin E and cyclin-dependent kinase 4 proteins due to overexpression of TIS21. Mol Carcinog. 1998;23(1):25–35.
Shuai Y, Ma Z, Liu W, et al. TEAD4 modulated LncRNA MNX1-AS1 contributes to gastric cancer progression partly through suppressing BTG2 and activating BCL2. Mol Cancer. 2020;19(1):6.
Wang W, Guo H, Zhou S, et al. Expression and clinical significance of B cell translocation gene 2 in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2021;14(4):475–83.
Imran M, Lim IK. Regulation of Btg2(/TIS21/PC3) expression via reactive oxygen species-protein kinase C-ΝFκΒ pathway under stress conditions. Cell Signal. 2013;25(12):2400–12.
Acknowledgements
The present study was supported by a Grant-in-Aid for Young Scientists (Grant No. 20K17609) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimizu, H., Katsurahara, K., Inoue, H. et al. NADPH Oxidase 2 Has a Crucial Role in Cell Cycle Progression of Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 29, 8677–8687 (2022). https://doi.org/10.1245/s10434-022-12384-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12384-5