Abstract
Background
Merkel cell carcinoma (MCC) is a rare cutaneous malignancy for which factors predictive of disease-specific survival (DSS) are poorly defined.
Methods
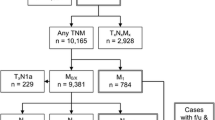
Patients from six centers (2005–2020) with clinical stage I–II MCC who underwent sentinel lymph node (SLN) biopsy were included. Factors associated with DSS were identified using competing-risks regression analysis. Risk-score modeling was established using competing-risks regression on a training dataset and internally validated by point assignment to variables.
Results
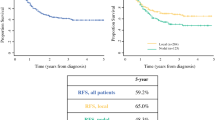
Of 604 patients, 474 (78.5%) and 128 (21.2%) patients had clinical stage I and II disease, respectively, and 189 (31.3%) had SLN metastases. The 5-year DSS rate was 81.8% with a median follow-up of 31 months. Prognostic factors associated with worse DSS included increasing age (hazard ratio [HR] 1.03, p = 0.046), male sex (HR 3.21, p = 0.021), immune compromise (HR 2.46, p = 0.013), presence of microsatellites (HR 2.65, p = 0.041), and regional nodal involvement (1 node: HR 2.48, p = 0.039; ≥2 nodes: HR 2.95, p = 0.026). An internally validated, risk-score model incorporating all of these factors was developed with good performance (AUC 0.738). Patients with ≤ 4.00 and > 4.00 points had 5-year DSS rates of 89.4% and 67.2%, respectively. Five-year DSS for pathologic stage I/II patients with > 4.00 points (n = 49) was 79.8% and for pathologic stage III patients with ≤ 4.00 points (n = 62) was 90.3%.
Conclusions
A risk-score model, including patient and tumor factors, based on DSS improves prognostic assessment of patients with clinically localized MCC. This may inform surveillance strategies and patient selection for adjuvant therapy trials.


Similar content being viewed by others
References
Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78(3):433–42. https://doi.org/10.1016/j.jaad.2017.12.001.
Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78(3):457-63.e2. https://doi.org/10.1016/j.jaad.2017.10.028.
Sridharan V, Muralidhar V, Margalit DN, et al. Merkel cell carcinoma: a population analysis on survival. J Natl Compr Canc Netw. 2016;14(10):1247–57. https://doi.org/10.6004/jnccn.2016.0134.
Farley CR, Perez MC, Soelling SJ, et al. Merkel cell carcinoma outcomes: does ajcc8 underestimate survival? Ann Surg Oncol. 2020;27(6):1978–85. https://doi.org/10.1245/s10434-019-08187-w.
Fitzgerald TL, Dennis S, Kachare SD, Vohra NA, Wong JH, Zervos EE. Dramatic increase in the incidence and mortality from merkel cell carcinoma in the United States. Am Surg. 2015;81(8):802–6. https://doi.org/10.1177/000313481508100819.
Harms KL, Healy MA, Nghiem P, et al. Analysis of prognostic factors from 9387 merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23(11):3564–71. https://doi.org/10.1245/s10434-016-5266-4.
Bichakjian C, Nghiem P, Johnson T, Wright C, Sober A. Merkel Cell Carcinoma (Chapter 46). AJCC Cancer Staging Manual. Springer; 2017:549–61.
Smith FO, Yue B, Marzban SS, et al. Both tumor depth and diameter are predictive of sentinel lymph node status and survival in Merkel cell carcinoma. Cancer. 2015;121(18):3252–60. https://doi.org/10.1002/cncr.29452.
Santamaria-Barria JA, Boland GM, Yeap BY, Nardi V, Dias-Santagata D, Cusack JC. Merkel cell carcinoma: 30-year experience from a single institution. Ann Surg Oncol. 2013;20(4):1365–73. https://doi.org/10.1245/s10434-012-2779-3.
Lim CS, Whalley D, Haydu LE, et al. Increasing tumor thickness is associated with recurrence and poorer survival in patients with Merkel cell carcinoma. Ann Surg Oncol. 2012;19(11):3325–34. https://doi.org/10.1245/s10434-012-2509-x.
Morand GB, Madana J, Da Silva SD, Hier MP, Mlynarek AM, Black MJ. Merkel cell carcinoma of the head and neck: poorer prognosis than non-head and neck sites. J Laryngol Otol. 2016;130(4):393–7. https://doi.org/10.1017/S0022215116000153.
Harounian JA, Molin N, Galloway TJ, et al. Effect of sentinel lymph node biopsy and LVI on Merkel cell carcinoma prognosis and treatment. Laryngoscope. 2021;131(3):E828–35. https://doi.org/10.1002/lary.28866.
Cook M, Baker K, Redman M, et al. Differential outcomes among immunosuppressed patients with Merkel cell carcinoma: impact of immunosuppression type on cancer-specific and overall survival. Am J Clin Oncol. 2019;42(1):82–8. https://doi.org/10.1097/COC.0000000000000482.
Butala AA, Jain V, Reddy VK, et al. Impact of tumor-infiltrating lymphocytes on overall survival in Merkel cell carcinoma. Oncologist. 2021;26(1):63–9. https://doi.org/10.1634/theoncologist.2020-0070.
Schwartz JL, Griffith KA, Lowe L, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol. 2011;29(8):1036–41. https://doi.org/10.1200/JCO.2010.33.4136.
Tarantola TI, Vallow LA, Halyard MY, et al. Prognostic factors in Merkel cell carcinoma: analysis of 240 cases. J Am Acad Dermatol. 2013;68(3):425–32. https://doi.org/10.1016/j.jaad.2012.09.036.
Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81(2):364–72. https://doi.org/10.1016/j.jaad.2019.03.027.
Kachare SD, Wong JH, Vohra NA, Zervos EE, Fitzgerald TL. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann Surg Oncol. 2014;21(5):1624–30. https://doi.org/10.1245/s10434-013-3434-3.
Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133(3):642–6. https://doi.org/10.1038/jid.2012.388.
Asgari MM, Sokil MM, Warton EM, Iyer J, Paulson KG, Nghiem P. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol. 2014;150(7):716–23. https://doi.org/10.1001/jamadermatol.2013.8116.
Iyer JG, Storer BE, Paulson KG, et al. Relationships among primary tumor size, number of involved nodes, and survival for 8044 cases of Merkel cell carcinoma. J Am Acad Dermatol. 2014;70(4):637–43. https://doi.org/10.1016/j.jaad.2013.11.031.
Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction. 2nd edn. Berlin: Springer; 2009. p. 219–59.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. https://doi.org/10.1080/01621459.1999.10474144.
StataCorp. Competing-risks regression. STATA. Accessed March 21, 2022, https://www.stata.com/manuals/ststcrreg.pdf
Rufibach K. Use of Brier score to assess binary predictions. J Clin Epidemiol. 2010;63(8):938–9. https://doi.org/10.1016/j.jclinepi.2009.11.009.
Hajian-Tilaki K. Receiver operating characteristic (ROC) Curve analysis for medical diagnostic test evaluation. Caspian J Int Med. 2013;4(2):627–35.
Liu X. Classification accuracy and cut point selection. Stat Med. 2012;31(23):2676–86. https://doi.org/10.1002/sim.4509.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–6. https://doi.org/10.1016/0197-2456(96)00075-x.
StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC; 2021.
Frohm ML, Griffith KA, Harms KL, et al. Recurrence and survival in patients with Merkel cell carcinoma undergoing surgery without adjuvant radiation therapy to the primary site. JAMA Dermatol. 2016;152(9):1001–7. https://doi.org/10.1001/jamadermatol.2016.1428.
Kubica AW, Brewer JD. Melanoma in immunosuppressed patients. Mayo Clin Proc. 2012;87(10):991–1003. https://doi.org/10.1016/j.mayocp.2012.04.018.
Nguyen AT, Luu M, Lu DJ, et al. Quantitative metastatic lymph node burden and survival in Merkel cell carcinoma. J Am Acad Dermatol. 2021;84(2):312–20. https://doi.org/10.1016/j.jaad.2019.12.072.
Lee JS, Durham AB, Bichakjian CK, et al. Completion lymph node dissection or radiation therapy for sentinel node metastasis in Merkel cell carcinoma. Ann Surg Oncol. 2019;26(2):386–94. https://doi.org/10.1245/s10434-018-7072-7.
Acknowledgments
The authors did not receive funding for the completion of this work. All authors have read and acknowledged the contents of this manuscript. C. Slingluff reports research funding to his University from Merck, Celldex, and GlaxoSmithKline; research support in kind to his University from Theraclion and 3M; Scientific Advisory Board role with Immatics (prior), and Curevac (planned); PI role for Polynoma with compensation to his University; and patent royalties as co-inventor of peptides for use in cancer vaccines (patents held by the UVA Licensing and Ventures Group), all outside the submitted work.
Author information
Authors and Affiliations
Contributions
MBF reports advisory board participation with Merck, Bristol Myers Squibb, Novartis, Array, Pulse Bioscience, and Sanofi, all outside the submitted work. G.M. Beasley reports relevant financial activities outside the submitted work as a member of the 2020 Regeneron Sanofi advisory board. V. Sondak reports personal fees from Array, Bristol Myers Squibb, Genentech, Merck, Novartis, Pfizer, Regeneron, Replimune, and research funding to his institution from Neogene Therapeutics, outside the submitted work. J.S. Zager reports advisory board participation with Merck, Amgen, and Sanofi Regeneron; speakers bureau participation with Array Biopharma and Sun Pharma; and research work with Amgen, Provectus, Castle Biosciences, and Delcath Systems, all outside the submitted work.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Shannon, A.B., Straker, R.J., Carr, M.J. et al. An Internally Validated Prognostic Risk-Score Model for Disease-Specific Survival in Clinical Stage I and II Merkel Cell Carcinoma. Ann Surg Oncol 29, 7033–7044 (2022). https://doi.org/10.1245/s10434-022-12201-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12201-z