Abstract
Introduction
Little is known about the societal burden of cancer surgical care in terms of out-of-pocket (OOP) costs. The current study sought to define OOP costs incurred by patients undergoing colorectal cancer resection.
Methods
Privately insured patients undergoing colorectal cancer resection between 2013 and 2017 were identified from the IBM MarketScan database. Total and OOP costs were calculated within 1 year prior to and 1 year post surgery. A multivariable linear regression model was used to estimate total OOP costs relative to patient demographic and clinical characteristics.
Results
Among 10,935 patients, 7289 (66.7%) had primary colon cancer while 3643 (33.3%) had rectal cancer. Median total costs were US$93,967 (IQR US$51027–168,251). Median OOP costs were US$4417 (IQR US$2519–6943), or 4.5% (IQR 2.2–8.1%) of total costs. OOP costs varied over the course of patient care; specifically, median OOP costs in the preoperative period were US$432 (IQR US$130–1452) versus US$2146 (IQR US$851–3525) in the perioperative period and US$969 (IQR US$327–2239) in the postoperative period. On multivariable analysis, receipt of chemotherapy (+US$1368, 95%CI +US$1211 to +US$1525) or radiotherapy (+US$842, 95% CI +US$626 to +US$1059) was associated with higher total OOP costs. Patients with a health maintenance organization (HMO) (−US$2119, 95% CI −US$2550 to −US$1689) or a point-of-service plan (−US$938, 95% CI −US$1385 to −US$491) had lower total OOP costs than patients with comprehensive insurance. In contrast, patients with a consumer-driven or a high-deductible health plan had considerably higher total OOP costs than patients with comprehensive insurance (+US$1400, 95% CI +US$972 to +US$1827 and +US$3243, 95% CI +US$2767 to +US$3717, respectively).
Conclusions
Privately insured colorectal cancer patients undergoing surgical resection pay a median of US$4417 in OOP costs, or 4.5% of total costs. OOP costs varied with receipt of chemotherapy or radiotherapy, region of residence, and insurance plan type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cancer represents the second leading cause of death in the USA, and costs associated with its treatment constitute a significant portion of total US healthcare spending.1,2 Specifically, costs associated with cancer care amounted to US$183 billion in 2015 and are projected to increase to US$246 billion by 2030.3 Despite the introduction of annual out-of-pocket (OOP) limits in 2014, cancer patients are responsible for paying a significant share of total cancer treatment costs.4 In 2018 alone, cancer patients paid US$5.8 billion OOP for cancer treatment.5 Such high OOP costs can often lead to financial hardship among cancer patients and survivors.6,7 In turn, cancer patients who experience financial hardship report worse health outcomes, including increased symptoms, decreased satisfaction with care, lower quality of life, and higher mortality.8,9,10,11 Notably, costs associated with cancer care do not impact all cancer patients equally. Patients of color, as well younger or less educated individuals are more likely to experience financial hardship following a cancer diagnosis.12
Colorectal cancer represents the third most common cancer and the second leading cause of cancer-related death in the USA.13,14 Its treatment commonly involves a combination of surgical resection, chemotherapy, and/or radiotherapy, resulting in substantial costs.15 As such, the associated OOP costs can result in financial strain among colorectal cancer patients and survivors.16,17 Most literature and public discourse on the financial hardship experienced by patients as a result of OOP costs has focused on the cost of drug treatments.18,19,20 Several studies have noted that OOP costs associated with cancer care spike immediately following diagnosis due to the high OOP costs relative to inpatient services, including surgery for potentially resectable cancers.21 OOP costs experienced by colorectal cancer patients who undergo surgical treatment remain poorly defined, however. As such, the objective of the current study is to determine total OOP costs among privately insured colorectal cancer patients incurred between 1 year prior to and 1 year following surgery. Additionally, we sought to identify the demographic and clinical factors associated with total OOP costs over this time period.
Methods
Patient Population
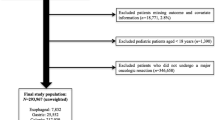
Patients with colorectal cancer who underwent surgical resection between 2013 and 2017 were identified from the commercial IBM MarketScan database, which contains medical and drug data retrieved from employers and health plans for more than 203 million individuals annually.22 Patients diagnosed with colon or rectal cancer were identified using the corresponding codes of the International Classification of Diseases, 9th and 10th revisions (ICD-9 and ICD-10) (Supplementary Table 1). Patients who underwent surgical resection of their primary tumor were identified using a combination of ICD-9, ICD-10, and Common Procedural Terminology (CPT) codes (Supplementary Table 2). Patients with an additional cancer diagnosis were excluded. Additionally, patients without continuous enrollment and drug coverage over the study period (from 12 months before surgery to 12 months following surgery) were excluded.
Variables and Outcomes
Variables of interest included patient age, sex, employment status (active full time, active part time, Consolidated Omnibus Budget Reconciliation Act (COBRA) continuee, etc.), health insurance type, and US Census Bureau region of residence, as well as whether patients resided in a Metropolitan Statistical Area (MSA). Patient health insurance types included comprehensive health coverage, exclusive provider organization (EPO), health maintenance organization (HMO), point-of-service (POS) plans, preferred provider organization (PPO), consumer-directed health plans (CDHP), and high-deductible health plans (HDHP). The 2010 Standards for delineating MSA require that the area must have at least one urbanized region of 50,000 or more inhabitants.23 In the current study, residence in a MSA was used as a proxy for residence in an urban setting. Preoperative comorbidities were accounted for using the Charlson Comorbidity Index (CCI).24,25,26 Information on treatment included year of surgery as well as receipt of chemotherapy and/or radiotherapy. Patients who received chemotherapy or radiotherapy were identified using the relevant revenue and CPT codes.
Total costs were calculated as the sum of all healthcare expenditures between 12 months prior to surgery and 12 months following surgery, including costs reimbursed by the primary insurance provider, out-of-pocket (OOP) costs incurred by the patient, as well as benefits received by other insurance carriers. OOP costs were calculated as the sum of deductibles, copayments, and coinsurance, as available in the IBM MarketScan commercial database. The proportion of OOP costs relative to total costs (OOP/total) was then calculated.
Total costs were the sum of expenditures associated with claims over four distinct time periods: the preoperative (from 1 year prior to surgery to 90 days prior to surgery), the perioperative (from 90 days prior to surgery to 30 days following surgery), the adjuvant (from 30 days following surgery to 90 days following surgery), and the surveillance period (from 90 days following surgery to 1 year following surgery). The definition of these four time periods was consistent with previous studies on OOP costs among cancer patients undergoing surgery.27
Statistical Analysis
Descriptive statistics are presented as median (interquartile range, IQR) for continuous variables and frequency (relative frequency, %) for categorical variables. Bivariate associations between cancer location (colon or rectum) and patient demographic or clinical characteristics were assessed using Kruskal–Wallis one-way analysis of variance for continuous variables and chi-squared tests for categorical variables. A multivariable linear regression model was developed to predict the overall OOP costs over the study period based on relevant patient demographic and clinical characteristics. All analyses were performed using SAS version 9.4. Statistical significance was assessed at α = 0.05.
Results
Patient Characteristics
Among 10,935 patients who underwent surgical resection for colorectal cancer between 2013 and 2017, median age was 55 years (IQR 50–59 years), and slightly more than one-half of patients were male (n = 5673, 51.9%) (Table 1). Approximately four in ten patients received chemotherapy (n = 5100, 46.6%), while fewer than two in ten patients underwent radiotherapy (n = 1877, 17.2%). Patients were most commonly from the South (n = 5122, 46.8%), followed by the Midwest (n = 2166, 19.8%), the Northeast (n = 2010, 18.4%), and the West (n = 1523, 13.9%). Additionally, the vast majority of patients resided in an MSA (n = 9000, 82.3%). At the time of surgery, more than five in ten patients were actively employed full time (n = 6057, 55.4%), while one in ten patients were retired (n = 1216, 11.1%). Only a small minority of patients were employed part time (n = 88, 0.8%); employment status was unknown in a subset of individuals (n = 3458, 31.6%). The most common health insurance type was PPO (n = 6549, 59.9%), followed by CDHP (n = 1153, 10.5%), HMO (n = 1130, 10.3%), and POS (n = 886, 8.1%).
The majority of patients underwent resection for colon cancer (n = 7289, 66.7%), while other individuals had resection for rectal cancer (n = 3643, 33.3%) (Table 1). Patients with rectal cancer were more often male (55.4% vs. 50.1%), as well as were more likely to receive chemotherapy (57.8% vs. 41.0%) or radiotherapy (45.2% vs. 3.1%) versus patients with colon cancer (all p < 0.001). Additionally, patients with rectal cancer more often underwent resection in the earlier years of the study period (2013: 37.1% vs. 27.3%; 2014: 25.7% vs. 20.2%; p < 0.001) compared with patients who had colon cancer.
Total and Out-of-Pocket Costs
Median total costs associated with all claims between 1 year preoperatively and 1 year postoperatively were US$93,967 (IQR US$51,027–168,251). Median OOP costs over the same period were US$4417 (IQR US$2519–6943), or 4.5% (IQR 2.2–8.1%) of total costs. Median total costs fluctuated considerably over the study period. Specifically, costs started at US$1921 (IQR US$543–11,336) in the preoperative period, peaked at US$44,723 (IQR US$32,398–65,575) in the perioperative period, and then decreased to US$9647 (IQR US$1031–24,714) in the adjuvant period, only to rise again to US$21,327 (IQR US$4749–60,591) in the postoperative period (Table 2). OOP costs followed a similar trend, going from US$432 (IQR US$130–1452) in the preoperative period to US$2146 (IQR US$851–3525) in the perioperative period, US$200 (IQR US$71–640) in the adjuvant period, and US$969 (IQR US$327–2239) in the postoperative period (Table 2; Fig. 1). Notably, OOP costs represented a higher proportion of total costs in the preoperative period (17.9%, IQR 7.6–36.1%) compared with all subsequent periods (perioperative: 4.5%, IQR 1.7–8.7%; adjuvant: 4.0%, IQR 1.1–13.8%; postoperative: 5.1%, IQR 1.8–14.5%).
When costs were stratified by service type, inpatient services were associated with much higher OOP costs compared with outpatient services (Table 2; Fig. 1). In particular, OOP costs associated with inpatient services amounted to US$1300 (IQR US$480–2453) in the preoperative period, US$1338 (IQR US$534–2440) in the perioperative period, US$433 (IQR, US$200–1203) in the adjuvant period, and US$845 (IQR US$291–2001) in the postoperative period. These OOP costs constituted a greater proportion of the total costs in the preoperative period (6.4%, IQR 2.1–14.2%) than in all subsequent periods (perioperative: 4.0%, IQR 1.6–8.0%; adjuvant: 2.0%, IQR 0.9–6.2%; postoperative: 2.5%, IQR 0.9–6.8%). OOP costs associated with outpatient services were lower and affected far fewer patients, amounting to US$400 (IQR US$126–1266) in the preoperative period, US$812 (IQR US$300–1672) in the perioperative period, US$190 (IQR US$70–576) in the adjuvant period, and US$847 (IQR US$303–1982) in the postoperative period. Similar to OOP costs associated with inpatient services, these OOP costs represented a greater proportion of total costs in the preoperative period (18.4%, IQR 8.0–36.7%) compared with all subsequent periods (perioperative: 9.2%, IQR 3.5–17.9%; adjuvant: 4.5%, IQR 1.2–14.6%; postoperative: 5.9%, IQR 2.1–15.4%).
Only a small minority of patients had OOP costs associated with receipt of chemotherapy or radiotherapy (Table 2). Among the patients who did have OOP costs, OOP costs associated with chemotherapy amounted to US$71 (IQR US$16–747) in the preoperative period, US$42 (IQR US$10–216) in the perioperative period, US$137 (IQR US$40–547) in the adjuvant period, and US$205 (IQR US$53–768) in the postoperative period. These OOP costs translate to 4.8% (IQR 1.2–13.3%) of total costs in the preoperative period, 10.0% (IQR 2.7–20.0%) in the perioperative period, 7.9% (IQR 2.8–18.4%) in the adjuvant period, and 4.0% (IQR 1.4–10.9%) in the postoperative period. OOP costs associated with radiotherapy were higher, amounting to US$374 (IQR US$140–921) in the preoperative period, US$321 (IQR US$106–843) in the perioperative period, US$446 (IQR US$100–1187) in the adjuvant period, and US$409 (IQR US$114–1060) in the postoperative period. These OOP costs correspond to 6.2% (IQR 1.9–12.9%) of total costs in the preoperative period, 4.4% (IQR 1.7–10.2%) in the perioperative period, 5.2% (IQR 1.2–12.8%) in the adjuvant period, and 4.7% (IQR 1.2–10.5%) in the postoperative period. Analyses stratified by cancer location (colon or rectum) demonstrated similar trends for both colon and rectal cancer patients (Table 3).
Multivariable Analysis
On multivariable analysis, several patient demographic and clinical characteristics were associated with total OOP costs between 1 year preoperatively and 1 year postoperatively (Table 4). In particular, older age (−US$9.66, 95% CI −US$19.10 to −US$0.21 per 1-year increase) and higher CCI score (+US$76.41, 95% CI +US$49.53 to +US$103.30 per 1-point increase) were associated with marginal changes in total OOP costs. In contrast, receipt of chemotherapy (+US$1367.70, 95% CI +US$1210.58 to +US$1524.82) and radiotherapy (+US$842.44, 95% CI +US$626.24–+US$1058.64) were both associated with much higher total OOP costs.
Additionally, there was substantial regional variability in total OOP costs. Patients from the Northeast (−US$1350.96, 95% CI −US$1537.88 to −US$1164.04), the Midwest (−US$605.27, 95% CI −US$787.74 to −US$422.79), or West (−US$453.04, 95% CI −US$660.86 to −US$245.21) all incurred considerably lower total OOP costs compared with patients from the South. Employment status was also associated with overall OOP costs, with early retirees and Medicare eligible retirees facing lower total OOP costs than full-time employees (−US$535.72, 95% CI −US$784.70 to −US$286.74 and −US$1108.20, 95% CI −US$1846.65 to −US$369.75, respectively). In contrast, COBRA continuees incurred substantially higher total OOP costs than full-time employees (+US$1705.62, 95% CI +US$311.0 to +US$3100.24). In addition, health insurance plan type was associated with total OOP costs. Specifically, patients with an EPO (−US$1598.89, 95% CI −US$2334.89 to −US$862.89), an HMO (−US$2119.09, 95% CI −US$2549.65 to −US$1688.54) or a POS plan (−US$938.09, 95% CI −US$1385.18 to −US$491.0) had lower total OOP costs than patients with a comprehensive health insurance plan. Conversely, patients with a CDHP or an HDHP plan had considerably higher total OOP costs compared with patients with a comprehensive health insurance plan (+US$1399.67$, 95% CI +US$972.04 to +US$1827.30 and +US$3242.58, 95% CI +US$2767.43 to +US$3717.72).
Discussion
Curative treatment of colorectal cancer requires a multidisciplinary approach that involves a combination of surgery, chemotherapy, and/or radiotherapy, leading to high treatment costs.28,29 In recent years, insurers have attempted to counteract the rising costs of cancer care by shifting a greater proportion of these costs to the patient through deductibles, copayments, and coinsurance.30 As a result, even insured colorectal cancer survivors often face significant financial hardship due to the direct and indirect costs of their treatment.19,31,32 As financial hardship is associated with worse outcomes for cancer patients, it is of utmost importance that patient–provider discussions on cancer treatment also include a discussion of the OOP costs associated with each treatment option.33 Existing evidence suggests that cancer patients would be comfortable with and open to such discussions.34 Despite this, patient–provider cost communication only rarely occurs in clinical practice.35 In the case of colorectal cancer patients, one obstacle to having meaningful and impactful patient–provider discussions on treatment costs is the lack of reliable estimates of OOP costs associated with curative treatment. Therefore, the current study is important because we assessed OOP costs between 1 year preoperatively and 1 year postoperatively in a large cohort of commercially insured colorectal cancer patients undergoing surgical resection. We found that patients pay a median of US$4417 in OOP costs over this period, or 4.5% of total costs. While OOP costs represented a lower proportion of total costs in the postoperative period, they were still significantly higher than the costs in the preoperative period. In addition, OOP costs varied widely relative to receipt of chemotherapy or radiotherapy, region of residence, employment status, and insurance plan type.
The main finding of the current study was that privately insured patients who underwent surgical resection for colorectal carcinoma spent a median of US$4417 in OOP costs between 1 year preoperatively and 1 year postoperatively. Somewhat unsurprisingly, OOP costs spiked in the perioperative period and gradually decreased in the postoperative period. However, postoperative OOP costs never returned to their preoperative levels (Table 2; Fig. 1). These findings are in line with a previous study by Dieguez et al., who noted a similar pattern of OOP spending among commercially insured colorectal cancer patients diagnosed in 2011.21 Specifically, Dieguez et al. reported that, following an initial peak associated with treatment, OOP costs did not return to baseline for as long as 3 years after the initial cancer diagnosis. Of note, the bulk of OOP costs over the study period was associated with inpatient services, while costs associated with outpatient services only represented a minor share of all OOP costs (Fig. 1). In a 2017 analysis of 1409 Medicare beneficiaries diagnosed with cancer, Narang et al. similarly reported that inpatient services accounted for a vast majority of total OOP costs.36 The current analysis was the first to note that radiotherapy and chemotherapy were also associated with meaningful OOP costs in the preoperative and postoperative period, respectively (Fig. 1). On multivariable analysis, receipt of chemotherapy and radiotherapy were both associated with a drastic increase in total OOP costs over the study period (Table 4). Since patients of lower socioeconomic status tend to place a disproportionate importance on costs when making treatment decisions, the OOP costs associated with radiotherapy and chemotherapy may be contributing to existing disparities in receipt of these treatment modalities for colorectal cancer treatment.37,38,39
Of note, several patient demographic and clinical characteristics were associated with OOP costs on multivariable analysis (Table 4). Specifically, older age was associated with a decrease in OOP costs, albeit a minor one. Previous studies had noted that younger cancer patients were particularly vulnerable to financial hardship following a cancer diagnosis.7,40 Results of the current study suggested that the financial impact of surgical treatment associated with colorectal cancer treatment among younger patients was mainly mediated by differences in employment and health insurance status, rather than by younger age itself. In particular, COBRA continuees faced higher OOP costs than full-time employees, who in turn incurred higher OOP costs than early retirees and Medicare eligible retirees. Additionally, there was a notable yet nonsignificant trend for higher OOP costs among part-time employees compared with full-time employees (Table 4). Colorectal cancer and its treatment are known to severely limit patient ability to work, resulting in lost wages and income, as well as disruptions in health insurance coverage.41,42 Results of the current study suggest that patients who are in less stable positions and are more prone to such disruptions face higher OOP costs when undergoing surgical treatment of colorectal cancer. Nevertheless, the single most important factor determining the amount of OOP costs incurred by colorectal cancer patients undergoing resection was their health insurance plan type. Notably, postoperative OOP costs accounted for a smaller proportion of overall costs than preoperative OOP costs (Table 2). As such, healthcare insurance was mostly effective at mitigating the financial impact of the surgical treatment of colorectal cancer. However, the amount of OOP costs paid by patients varied widely based on the specific insurance plan type. Specifically, patients with a CDHP or HDHP paid as much as US$1400 and US$3242 more, respectively, than patients with a comprehensive plan, who in turn paid US$2119 more than patients with an HMO plan (Table 4). Of note, HDHPs have recently grown in popularity, and as many as 51% of all US workers were enrolled in one in 2019.43 As such, OOP costs associated with the surgical treatment of colorectal cancer can be expected to have an increasingly large impact on a growing number of patients.
Findings from the current study may inform initiatives aimed at mitigating the financial impact of surgical treatment of colorectal cancer patients. Specifically, the fact that OOP costs remain elevated well beyond the initial spike associated with surgical treatment suggested that patient–provider cost discussions should not stop at the treatment selection phase, but should also include the long-term financial repercussions of treatment (Fig. 1). Additionally, the type of health insurance plan in which colorectal cancer patients were enrolled was the single most important factor in determining the amount of OOP costs incurred, as had been similarly reported relative to other cancer patient populations (Table 4).44,45 As such, colorectal cancer patients should be counseled about the options available to switch to a more favorable health insurance plan during the next available open enrollment period. Should patients decide to remain enrolled in a HDHP, the high deductibles associated with such plans may be at least partially offset by participation in a health savings account (HSA). Of note, however, significant race- and income-based disparities exist in HSA participation, to the point that not all patients may be able to benefit from the financial protection a HSA offers.46 Several factors other than treatment options have an impact on OOP costs associated with cancer care, including the setting in which the treatment is provided and the involvement of out-of-network providers at in-network hospitals.2,47,48 Transparency in patient–provider cost discussions about such factors would greatly limit their impact on the OOP costs associated with surgical treatment of colorectal cancer.
Several limitations should be considered when interpreting the results of the current study. The IBM MarketScan commercial database is based on a large, nonrandom convenience sample that may lead to a sample bias (i.e., the South portion of the USA overrepresented), leading to lack of generalizability.49 As with all studies relying on administrative databases, only variables associated with specific ICD or CPT codes could be examined. As such, no information on tumor stage or guideline-concordant care was available. This shortcoming is particularly relevant because associations between factors such as region of residence and OOP costs may be at least in part explained by differences in tumor stage at presentation. Additionally, the current study only included commercially insured individuals, who represent approximately two-thirds of the US population.50 As such, results of the current study cannot be extrapolated to patients who are insured through a public plan or are uninsured. Moreover, information on monthly premiums paid by patients was not available. Since premiums represent a portion of OOP costs for commercially insured individuals and differ based on plan type, we were limited in our ability to assess the financial OOP burden associated with each plan type. Of note, the current study only considered direct OOP costs associated with cancer treatment. However, indirect costs associated with transportation or lost income may be equally as impactful on patient finances.51 Lastly, patients included in the current study underwent surgery between 2013 and 2017. The ongoing coronavirus disease 2019 (COVID-19) pandemic is likely to have had a large impact on the costs of cancer care by means of delayed or skipped cancer screening, postponed or missed cancer treatment, and insurance coverage disruptions.52,53 The current study could not capture these recent changes.
In conclusion, privately insured patients who underwent surgical resection of colorectal cancer paid a median of US$4417 in OOP costs within 1 year prior and 1 year after surgery, which corresponded to 4.5% of total costs. Notably, OOP costs varied widely with receipt of chemotherapy or radiotherapy, region of residence, employment status, and health insurance plan type. By providing estimates of excess OOP costs associated with several patient- and treatment-level factors, results of the current study may inform patient–provider discussions on treatment preferences. Specifically, these data may assist patients in assessing the cost–benefit ratio of various treatment options, plan around the expenses associated with cancer care, and be promptly referred to financial navigation services if difficulties with payments are expected. The data highlight the need for policies to ensure that OOP costs do not dissuade cancer patients from seeking optimal care.
References
Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325(18):1829–30.
American Cancer Society Cancer Action Network. The Costs of Cancer. 2020 Edition. 2020.
Mariotto AB, Enewold L, Zhao J, et al. Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1304–12.
Dixon MS, Cole AL, Dusetzina SB. Out-of-pocket spending under the affordable care act for patients with cancer. Cancer J. 2017;23(3):175–80.
Agency for Healthcare Research and Quality. Medical Expenditure Panel Survery 2018. https://meps.ahrq.gov/mepsweb/. Accessed 15 Oct 2021.
Altice CK, Banegas MP, Tucker-Seeley RD, et al. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2017; 109(2).
Zheng Z, Jemal A, Han X, et al. Medical financial hardship among cancer survivors in the United States. Cancer. 2019;125(10):1737–47.
Lathan CS, Cronin A, Tucker-Seeley R, et al. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34(15):1732–40.
Chino F, Peppercorn J, Taylor DH Jr, et al. Self-reported financial burden and satisfaction with care among patients with cancer. Oncologist. 2014;19(4):414–20.
Huang IC, Bhakta N, Brinkman TM, et al. Determinants and consequences of financial hardship among adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. J Natl Cancer Inst. 2019;111(2):189–200.
Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34(9):980–6.
Han X, Zhao J, Zheng Z, et al. Medical financial hardship intensity and financial sacrifice associated with cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2020;29(2):308–17.
Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–64.
American Cancer Society. Key Statistics for Colorectal Cancer Jan, 12 2021, 2021. https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html. Accessed 6 July 2021.
Orangio GR. The economics of colon cancer. Surg Oncol Clin N Am. 2018;27(2):327–47.
LaRocca CJ, Li A, Lafaro K, et al. The impact of financial toxicity in gastrointestinal cancer patients. Surgery. 2020;168(1):167–72.
Rice DR, Farooq A, Hyer JM, et al. Health expenditures and financial burden among patients with major gastrointestinal cancers relative to other common cancers in the United States. Surgery. 2020;167(6):985–90.
Bach PB, Saltz L, Wittes RE. In Cancer Care, Cost Matters 2021. https://www.nytimes.com/2012/10/15/opinion/a-hospital-says-no-to-an-11000-a-month-cancer-drug.html. Accessed 18 Jan 2022.
Shankaran V, Jolly S, Blough D, et al. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: a population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608–14.
Seal BS, Sullivan SD, Ramsey S, et al. Medical costs associated with use of systemic therapy in adults with colorectal cancer. J Manag Care Pharm. 2013;19(6):461–7.
Dieguez G, Ferro C, Pyenson BS. A Multi-Year Look at the Cost Burden of Cancer Care 2017. https://www.accc-cancer.org/docs/Documents/advocacy/pdf/burden-of-cancer-care-final-report-milliman-2017.
International Business Machines Corporation. IBM MarketScan Research Databases 2021. https://www.ibm.com/products/marketscan-research-databases/databases. Accessed 6 Oct 2021.
National Archives and Records Administration. 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas June 28, 2010. https://www.govinfo.gov/content/pkg/FR-2010-06-28/pdf/2010-15605.pdf. Accessed 11 Oct 2021.
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82.
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Zheng F, Huang Y, Wright J, et al. Out of pocket costs for patients undergoing thyroid surgery. Ann Surg. 2021.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Colon Cancer. Version 3.2021. 2021.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Rectal Cancer. Version 2.2021. 2021.
Claxton G, Levitt L, Rae M, et al. Increases in cost-sharing payments continue to outpace wage growth. June 15, 2018. https://www.healthsystemtracker.org/brief/increases-in-cost-sharing-payments-have-far-outpaced-wage-growth/.
Hanly P, Maguire R, Ceilleachair AO, et al. Financial hardship associated with colorectal cancer survivorship: the role of asset depletion and debt accumulation. Psychooncology. 2018;27(9):2165–71.
Regenbogen SE, Veenstra CM, Hawley ST, et al. The personal financial burden of complications after colorectal cancer surgery. Cancer. 2014;120(19):3074–81.
Rai A, Zheng Z, Zhao J, et al. Patient-provider discussions about out-of-pocket costs of cancer care in the U.S. Am J Prev Med. 2020;59(2):228–36.
Bullock AJ, Hofstatter EW, Yushak ML, et al. Understanding patients’ attitudes toward communication about the cost of cancer care. J Oncol Pract. 2012;8(4):e50–8.
Shih YT, Chien CR. A review of cost communication in oncology: patient attitude, provider acceptance, and outcome assessment. Cancer. 2017;123(6):928–39.
Narang AK, Nicholas LH. Out-of-pocket spending and financial burden among medicare beneficiaries with cancer. JAMA Oncol. 2017;3(6):757–65.
Wong YN, Egleston BL, Sachdeva K, et al. Cancer patients’ trade-offs among efficacy, toxicity, and out-of-pocket cost in the curative and noncurative setting. Med Care. 2013;51(9):838–45.
Zhong X, Guo Z, Gao P, et al. Racial/ethnic disparities in the adjuvant chemotherapy of locally advanced colon cancer patients. J Surg Res. 2018;228:27–34.
Ofshteyn A, Bingmer K, Dorth J, et al. Disparities in neoadjuvant radiation dosing for treatment of rectal cancer. Am J Surg. 2020;220(4):987–92.
Yabroff KR, Dowling EC, Guy GP Jr, et al. Financial hardship associated with cancer in the United States: findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34(3):259–67.
Yabroff KR, Reeder-Hayes K, Zhao J, et al. Health insurance coverage disruptions and cancer care and outcomes: systematic review of published research. J Natl Cancer Inst. 2020;112(7):671–87.
Yuan CM, Wang CC, Wu WT, et al. Risk factors for return to work in colorectal cancer survivors. Cancer Med. 2021;10(12):3938–51.
Price S. 51% of the U.S. workforce enrolled in high-deductible health plans, which may leave some underinsured January 25, 2021. https://www.valuepenguin.com/enrollment-changes-to-high-definition-health-insurance-plans#nation. Accessed 25 Oct 2021.
Fu SJ, Rose L, Dawes AJ, et al. Out-of-pocket costs among patients with a new cancer diagnosis enrolled in high-deductible health plans vs traditional insurance. JAMA Netw Open. 2021;4(12):e2134282.
Leopold C, Wagner AK, Zhang F, et al. Total and out-of-pocket expenditures among women with metastatic breast cancer in low-deductible versus high-deductible health plans. Breast Cancer Res Treat. 2018;171(2):449–59.
Ellison J, Shafer P, Cole MB. Racial/ethnic and income-based disparities in health savings account participation among privately insured adults. Health Aff (Millwood). 2020;39(11):1917–25.
Robinson JC, Whaley CM, Brown TT. Price differences to insurers for infused cancer drugs in hospital outpatient departments and physician offices. Health Aff (Millwood). 2021;40(9):1395–401.
American Cancer Society Cancer Action Network. Survey: Cancer Patients and Survivors Alter or Delay Care Due to Insurance Barriers December 5, 2019, 2019. https://www.fightcancer.org/releases/survey-cancer-patients-and-survivors-alter-or-delay-care-due-insurance-barriers. Accessed 19 Jan 2022.
IBM Watson Health. IBM MarketScan Research Databases for life sciences researchers 2021. https://www.ibm.com/downloads/cas/OWZWJ0QO. Accessed 19 Jan 2022.
Keisler-Starkey KB, L.N. Health Insurance Coverage in the United States: 2019. Current Population Reports. U.S. Census Bureau 2020.
Yabroff KR, Warren JL, Knopf K, et al. Estimating patient time costs associated with colorectal cancer care. Med Care. 2005;43(7):640–8.
Kaufman HW, Chen Z, Niles J, et al. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3(8):e2017267.
Dorn S. The Covid-19 Pandemic and Resulting Economic Crash Have Caused the Greatest Health Insurance Losses in American History July 17, 2020, 2020. https://www.familiesusa.org/resources/the-covid-19-pandemic-and-resulting-economic-crash-have-caused-the-greatest-health-insurance-losses-in-american-history/. Accessed 20 Oct 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paro, A., Hyer, J.M., Shaikh, C.F. et al. Financial Impact of Out-of-Pocket Costs Among Patients Undergoing Resection for Colorectal Carcinoma. Ann Surg Oncol 29, 5387–5397 (2022). https://doi.org/10.1245/s10434-022-11755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11755-2