Abstract
Background
Injury to the artery of Adamkiewicz (AKA) during surgery may lead to spinal cord ischemia and severe neurologic complications. Posterior mediastinal tumors may be adjacent to AKA, but data on preoperative visualization of AKA in children are rare. This study analyzed the importance of identifying the AKA preoperatively by spinal digital subtraction angiography (DSA) in children with posterior mediastinal tumors for therapeutic procedure.
Methods
Between 2002 and 2021, 36 children with posterior mediastinal tumors were evaluated for surgery at the authors’ clinic. In 10 children with left-sided or bilateral tumor located at vertebral levels T8 to L1, spinal DSA was performed during preoperative workup to assess AKA. The patient and tumor characteristics as well as the diagnostic and therapeutic procedures were analyzed.
Results
The median age of the 10 children at examination was 69 months (range, 16–217 months). Three of the children were younger than 2 years. The tumor entities were neuroblastoma, ganglioneuroblastoma, ganglioneuroma, local relapse of a hepatocellular carcinoma, and neurofibroma. The AKA was identified in all cases, and proximity to the tumor was detected in four patients, three of whom had their planned surgery changed to irradiation. No complications occurred during spinal DSA or surgery.
Conclusions
In posterior mediastinal pediatric tumors, spinal DSA is a safe and reliable method for preoperative visualization of the AKA. It can show proximity to the tumor and guide the local therapy, thereby avoiding critical intra- and postoperative situations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Tumors in the posterior mediastinum in children account for approximately one third of all mediastinal tumors.1 They comprise a variety of types,2,3 but approximately 90% are of neurogenic origin, with neuroblastoma as the most common tumor.4,5
Neurogenic tumors originate from the sympathetic nervous system and peripheral nerves. Therefore, they often are located paravertebrally in the vicinity of the vascular system that ensures blood supply to the spinal cord. Injury to the vasculature during surgery may result in decreased spinal cord perfusion and ischemia, leading to paraplegia and paraparesis.6
Of particular importance is the artery of Adamkiewicz (AKA).7,8 The AKA is located between vertebral levels T3 and L5, but in 89% of patients, it is located between levels T8 and L1, and 76.6% of AKAs arise from the left side.9
Specific reference is made to the AKA in the International Neuroblastoma Risk Group Staging System (INRGSS).10 Because of possible injury to the AKA during surgery, infiltration of the costovertebral junction between vertebral levels T9 and T12 is defined as an image-defined risk factor (IDRF).11 However, reports on surgical complications in cooperative neuroblastoma studies and metanalyses provide only overall incidences without giving more specific details for posterior mediastinal tumors, particularly concerning injury to the AKA.12,13,14,15,16,17,18,19
Ultimately, data on impairment of neurologic function due to spinal cord ischemia after surgery for posterior mediastinal tumors in children are limited to case reports.20,21,22,23 Even less information is available on the demonstration of AKA to guide the surgical procedure. After experiencing a postoperative neurologic deficit in a child after surgery of posterior mediastinal tumors, Boglino et al.20 and Nordin et al.23 recommended that spinal diagnostic subtraction angiography (DSA) be performed when the AKA is at risk, but only Nordin et al.23 described spinal DSA performed in two children.
This study presents the largest case series of preoperative spinal DSA in children with solid tumors in the posterior mediastinum to date. We discuss the criteria for applying the method and describe the impact of its implementation on an unselected patient cohort.
Methods
Study Design and Patients
The patient records of all the children evaluated for surgery of posterior mediastinal tumors at our clinic from 2002 to 2021 were screened. The parameters analyzed were patient age, tumor type and location, spinal DSA, surgery, diagnostics and surgery complications, and outcome. The patient data are presented as median and range. The study was approved by the Ethical Committee of the Medical Faculty of the University of Tuebingen and the University Hospital Tuebingen, Germany (ref. 947/2020BO2).
Selective Spinal DSA
All the children had already received magnetic resonance imaging (MRI) or computed tomography (CT) scan before spinal DSA for diagnosis and planning for surgery. Spinal DSA was performed if the tumor extended to vertebral levels T8 to L1 on the left side. For spinal DSA, we used the biplane Siemens Artis zee with the as40HDR HDR flat detector system, the MEGALIC Cat Plus x-ray tube, and Automatic Exposure Control (AED; Siemens Healthineers, Erlangen, Germany). Spinal angiography was performed with the patient under general anesthesia using a pediatric femoral sheath and 4-French diagnostic catheters.
Results
Patient Characteristics
A total of 36 patients with posterior mediastinal tumors were evaluated for surgery. All the patients were of caucasian ethnicity. In 10 patients (4 girls and 6 boys), the left-sided or bilateral tumor extended into vertebral levels T8 to L1. Spinal DSA was performed for these patients. The tumors were neuroblastoma (n = 4), ganglioneuroblastoma (n = 2), ganglioneuroma (n = 2), local relapse of a hepatocellular carcinoma (n = 1), and neurofibroma (n = 1). The median age at the time of spinal DSA was 69 months (range, 16–217 months), and three of the children were younger than 2 years.
Tumor and AKA Location
The tumors were located between vertebral levels T3 and L2 (Table 1) on the left side or bilaterally. The AKA was detected in all cases. It originated at T7 to L2/3, most often at L1 (n = 3). Seven AKAs were located on the left side and three on the right side. Tumor and AKA were at the same level and side in four patients (patients 4, 6, 7, and 9) and at a different level or side in six patients (patients 1, 2, 3, 5, 8, and 10) (Table 1).
Therapy and Outcome
Surgery was performed as planned for five patients with differing locations of AKA and tumor (shown for patient 5; Fig. 1). The tumor of one patient (patient 3) showed spontaneous regression, so that in accordance with the treatment protocol, local therapy was not applied. In the four children with coincident location of the origin of the AKA and tumor, the individual risk assessment led to different therapeutic strategies.
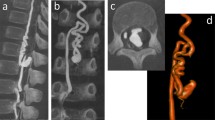
Left-sided, thoracic ganglioneuroma in a 5-year-old girl (patient 5). (a) Coronar and (b) sagittal magnetic resonance (MR) images. The tumor is located from T6 to T8. (c) Coronar and (d) sagittal images of selective spinal angiography. The origin of the artery of Adamkiewicz at level L1 is on the left. (e) Clamshell incision for tumor resection. (f) Paravertebrally located tumor. (g) Situs after tumor resection.
In three children, surgery was considered too risky, and the treatment method was changed. One patient with local relapse of a hepatocellular carcinoma (Fig. 2) received proton beam therapy, and one patient with neuroblastoma (Fig. 3) underwent conventional radiotherapy. One patient with thoracoabdominal neuroblastoma (patient 9) underwent surgery on the abdominal part of the tumor. At this writing, irradiation of the thoracic part is scheduled. The decision was made by the interdisciplinary tumor board consisting of pediatric radiologists, pediatric oncologists, and pediatric surgeons.
Left-sided, paravertebral local relapse of hepatocellular carcinoma in an 18-year-old girl (patient 4). (a) Coronar and (b) axial MR images. The tumor is located from T12 to L2. (c) Coronar and (d) sagittal images of selective spinal angiography. The origin of the artery of Adamkiewicz is at level L1 on the left. Surgery was abandoned, and proton beam therapy was performed.
Left-sided, thoracic neuroblastoma in a 6-year-old boy (patient 6). (a) Coronar and (b) axial magnetic resonance (MR) images. The tumor is located from T7 to T10. (c) Coronar and (d) sagittal images of selective spinal angiography. The origin of the artery of Adamkiewicz is at level T9 on the left. Surgery was abandoned, and external irradiation was performed.
The patient with neurofibroma underwent surgery. It was anticipated that the neurofibroma could be excised with the capsule intact, and thus with a low risk of injury to the AKA. The tumor was successfully removed. No complications occurred during either DSA or surgery.
Tumor recurrence was not observed in the patients who received local therapy (6/10) with a median follow-up period of 25 months (range, 2–94 months) (Table 2). One patient was lost to follow-up evaluation.
Discussion
This report describes our experience with preoperative selective spinal DSA in surgery for solid tumors of the posterior mediastinum in children and its impact on the therapeutic strategy. In 10 of 36 patients who underwent surgery for posterior mediastinal tumor, the tumor was located left-sided or bilaterally and extended into the vertebral levels of T8 to L1, so spinal DSA was performed. In four patients, the AKA originated at the same level and side as the tumor. To avoid injury to the AKA, the planned surgery was not performed for three of the patients. No complications were associated with surgery or spinal DSA.
Nordin et al.23 successfully and safely performed spinal DSA in two children with posterior mediastinal tumors. A systematic review of 11 studies comprising 87 patients receiving spinal DSA for diagnosis, preoperative planning, and therapy in pediatric spinal vascular pathologies reported two post-interventional complications (scrotal swelling and procedure-related subarachnoid hemorrhage).24 The data support a positive assessment of spinal DSA for children in general.
Spinal DSA is an invasive method associated with radiation exposure. Therefore, its application should be carefully assessed. We limit it to situations that indicate an increased risk of injury to the AKA, which is extension of the tumor to vertebral levels T8 to L1 and location on the left side.
Vertebral levels T8 to L1 differ from vertebral levels T9 to T12, which are the boundaries in the INRGSS for image-defined risk factors concerning infiltration of the costovertebral junction.10 A meta-analysis has shown that 7.3% of the AKAs arise at T8 and 6.9% at L1.9 Interestingly, in our cohort, the AKA appeared at L1 in three patients, for whom we thus would not have performed DSA if we had not included this vertebral level.
For diagnostic workup, we applied the diagnostic gold standard of spinal DSA and did not use MRI or CT angiography, which are used for AKA visualization in adults.25,26,27,28,29 Only 64-section CT is described for children.30 However, this method is associated with a potentially higher radiation dose due to the large field of view necessary for diagnosis and the possible misinterpretation of the anterior medullary vein as the AKA in case of non-optimal contrast agent timing. The latter risk exists particularly for children, in whom the vessels generally are smaller than in adults.30 Noninvasive methods developed for the demonstration of AKA in adults may be applicable, but to date have not been validated for children.
With modern techniques, spinal angiography can be performed as a very targeted diagnostic procedure, with low radiation exposure focused on the affected vertebral segments and starting on the left side. The relatively high incidence of complications cited by Ou et al.30 is based on data reported more than 20 years ago that do not reflect the current clinical and technical standard. Due to the precise localization of the origin of AKA in relation to the tumor, spinal DSA remains the diagnostic method of choice.
Conclusions
Spinal DSA can be safely and reliably applied for preoperative AKA demonstration in children with a posterior mediastinal tumor. It allows a valid assessment of the surgical risk and thus may prevent an injury to the AKA. Performing DSA for left-sided tumors extending to vertebral levels T8 to L1, the most common region of AKA location, avoids its unnecessary use.
References
Sairanen H, Leijala M, Louhimo I. Primary mediastinal tumors in children. Eur J Cardiothorac Surg. 1987;1:148–51.
Grosfeld JL, Weinberger M, Kilman JW, Clatworthy HW Jr. Primary mediastinal neoplasms in infants and children. Ann Thorac Surg. 1971;12:179–90.
Gun F, Erginel B, Unuvar A, Kebudi R, Salman T, Celik A. Mediastinal masses in children: experience with 120 cases. Pediatr Hematol Oncol. 2012;29:141–7.
Saenz NC, Schnitzer JJ, Eraklis AE, et al. Posterior mediastinal masses. J Pediatr Surg. 1993;28:172–6.
Jaggers J, Balsara K. Mediastinal masses in children. Semin Thorac Cardiovasc Surg. 2004;16:201–8.
Sliwa JA, Maclean IC. Ischemic myelopathy: a review of spinal vasculature and related clinical syndromes. Arch Phys Med Rehabil. 1992;73:365–72.
Bosmia AN, Hogan E, Loukas M, Tubbs RS, Cohen-Gadol AA. Blood supply to the human spinal cord: part I. Anatomy and hemodynamics. Clin Anat. 2015;28:52–64.
Lindeire S, Hauser JM. Anatomy, Back, Artery of Adamkiewicz. StatPearls. Treasure Island (FL); 2020.
Taterra D, Skinningsrud B, Pekala PA, et al. Artery of Adamkiewicz: a meta-analysis of anatomical characteristics. Neuroradiology. 2019;61:869–80.
Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27:298–303.
McCarville MB. Imaging neuroblastoma: what the radiologist needs to know. Cancer Imaging. 2011;11(Spec No A[1a]):S44-47.
Simon T, Hero B, Benz-Bohm G, von Schweinitz D, Berthold F. Review of image defined risk factors in localized neuroblastoma patients: results of the GPOH NB97 trial. Pediatr Blood Cancer. 2008;50:965–9.
Monclair T, Mosseri V, Cecchetto G, De Bernardi B, Michon J, Holmes K. Influence of image-defined risk factors on the outcome of patients with localised neuroblastoma. A report from the LNESG1 study of the European International Society of Paediatric Oncology Neuroblastoma Group. Pediatr Blood Cancer. 2015;62:1536–42.
Parhar D, Joharifard S, Lo AC, Schlosser MP, Daodu OO. How well do image-defined risk factors (IDRFs) predict surgical outcomes and survival in patients with neuroblastoma? A systematic review and meta-analysis. Pediatr Surg Int. 2020;36:897–907.
Yoneda A, Nishikawa M, Uehara S, et al. Can image-defined risk factors predict surgical complications in localized neuroblastoma? Eur J Pediatr Surg. 2016;26:117–22.
Pohl A, Erichsen M, Stehr M, et al. Image-defined risk factors correlate with surgical radicality and local recurrence in patients with neuroblastoma. Klin Padiatr. 2016;228:118–23.
Irtan S, Brisse HJ, Minard-Colin V, et al. Image-defined risk factor assessment of neurogenic tumors after neoadjuvant chemotherapy is useful for predicting intra-operative risk factors and the completeness of resection. Pediatr Blood Cancer. 2015;62:1543–9.
Liu T, Lv Z, Xu W, Liu J, Sheng Q. Role of image-defined risk factors in predicting surgical complications of localized neuroblastoma. Pediatr Surg Int. 2020;36:1167–72.
Fischer J, Pohl A, Volland R, et al. Complete surgical resection improves outcome in INRG high-risk patients with localized neuroblastoma older than 18 months. BMC Cancer. 2017;17:520.
Boglino C, Martins AG, Ciprandi G, Sousinha M, Inserra A. Spinal cord vascular injuries following surgery of advanced thoracic neuroblastoma: an unusual catastrophic complication. Med Pediatr Oncol. 1999;32:349–52.
Furak J, Geczi T, Tiszlavicz L, Lazar G. Postoperative paraplegia after resection of a giant posterior mediastinal tumour. Importance of the blood supply in the upper spinal cord. Interact Cardiovasc Thorac Surg. 2011;12:855–6.
De Bernardi B, Gambini C, Haupt R, et al. Retrospective study of childhood ganglioneuroma. J Clin Oncol. 2008;26:1710–6.
Nordin AB, Fallon SC, Jea A, Kim ES. The use of spinal angiography in the management of posterior mediastinal tumors: case series and review of the literature. J Pediatr Surg. 2013;48:1871–7.
Goethe E, LoPresti MA, Kan P, Lam SK. The role of spinal angiography in the evaluation and treatment of pediatric spinal vascular pathology: a case series and systematic review. Childs Nerv Syst. 2020;36:325–32.
Kawaharada N, Morishita K, Hyodoh H, et al. Magnetic resonance angiographic localization of the artery of Adamkiewicz for spinal cord blood supply. Ann Thorac Surg. 2004;78:846–51.
Takase K, Sawamura Y, Igarashi K, et al. Demonstration of the artery of Adamkiewicz at multi- detector row helical CT. Radiology. 2002;223:39–45.
Backes WH, Nijenhuis RJ. Advances in spinal cord MR angiography. AJNR Am J Neuroradiol. 2008;29:619–31.
Uotani K, Yamada N, Kono AK, et al. Preoperative visualization of the artery of Adamkiewicz by intra-arterial CT angiography. AJNR Am J Neuroradiol. 2008;29:314–8.
Yoshioka K, Tanaka R, Takagi H, et al. Ultra-high-resolution CT angiography of the artery of Adamkiewicz: a feasibility study. Neuroradiology. 2018;60:109–15.
Ou P, Schmit P, Layouss W, Sidi D, Bonnet D, Brunelle F. CT angiography of the artery of Adamkiewicz with 64-section technology: first experience in children. AJNR Am J Neuroradiol. 2007;28:216–9.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
U. Ernemann and J. Fuchs contributed equally to this work and share senior authorship.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmidt, A., Hempel, JM., Ellerkamp, V. et al. The Relevance of Preoperative Identification of the Adamkiewicz Artery in Posterior Mediastinal Pediatric Tumors. Ann Surg Oncol 29, 493–499 (2022). https://doi.org/10.1245/s10434-021-10381-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10381-8