Abstract
Background
Sarcopenia was previously linked to clinical outcomes for several cancer types, including esophageal cancer (EC), but most studies only measured the quantity of skeletal muscle mass. We aim to assess the clinical significance of evaluating the quantity and quality of skeletal muscle in patients with EC who underwent neoadjuvant chemotherapy (NAC) followed by esophagectomy.
Methods
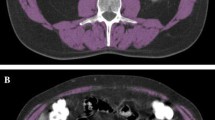
We included 333 consecutive patients with EC who underwent NAC followed by esophagectomy. The psoas muscle index (PMI) and intracellular muscle adipose tissue content (IMAC) were measured by computed tomography. We defined low PMI combined with high IMAC as severe sarcopenia, and assessed its impact on clinical outcomes.
Results
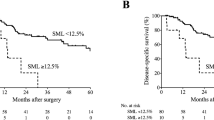
Thirty-seven patients (11.1%) had severe sarcopenia. Compared with patients without severe sarcopenia, those with severe sarcopenia showed a significantly worse NAC response rate (54.1% vs 74.7%; P = 0.008), worse pathological response rate (24.3% vs 40.2%, P = 0.061), higher morbidity rate (67.6% vs 38.5%; P = 0.001), particularly for pneumonia (32.4% vs 14.9% P = 0.007) and expectoration disorder (37.8% vs 13.5% P < 0.001), and unfavorable survival (3-year overall survival rate: 54.1% vs 66.6% P = 0.027). Multivariable analysis of overall survival showed that severe sarcopenia (HR 1.68, P = 0.025) and cT (HR 1.52, P = 0.032) were independent prognostic factors of poor outcome.
Conclusions
PMI combined with IMAC represents a new criterion for sarcopenia that might be useful for predicting NAC response, postoperative complications, and long-term survival in patients with EC undergoing multidisciplinary treatments.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author, Tomoki Makino. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
References
Gertler R, Stein HJ, Langer R, et al. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: evaluation of the New Union Internationale Contre le Cancer/American Joint Cancer Committee staging system. Ann Surg. 2011;253(4):689–98.
Yamashita K, Makino T, Miyata H, et al. Postoperative infectious complications are associated with adverse oncologic outcomes in esophageal cancer patients undergoing preoperative chemotherapy. Ann Surg Oncol. 2016;23(6):2106–14.
Wu PC, Posner MC. The role of surgery in the management of oesophageal cancer. Lancet Oncol. 2003;4(8):481–8.
Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190(5):562–72.
Japanese Classification of Esophageal Cancer. 11th Edition: part I. Esophagus. 2017;14(1):1–36.
Japanese Classification of Esophageal Cancer. 11th Edition: part II and III. Esophagus. 2017;14(1):37–65.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
Miyamoto Y, Baba Y, Sakamoto Y, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22(8):2663–8.
Onesti JK, Wright GP, Kenning SE, et al. Sarcopenia and survival in patients undergoing pancreatic resection. Pancreatology. 2016;16(2):284–9.
Go SI, Park MJ, Song HN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Supportive Care Cancer. 2016;24(5):2075–84.
Ishida T, Makino T, Yamasaki M, et al. Impact of measurement of skeletal muscle mass on clinical outcomes in patients with esophageal cancer undergoing esophagectomy after neoadjuvant chemotherapy. Surgery. 2019;166(6):1041–7.
Deng HY, Zha P, Peng L, Hou L, Huang KL, Li XY. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: a comprehensive systematic review and meta-analysis. Dis Esophagus. 2019;32(3).
Fukuda Y, Asaoka T, Eguchi H, et al. Clinical Impact of Preoperative sarcopenia on the postoperative outcomes after pancreas transplantation. World J Surg. 2018;42(10):3364–71.
Ojima Y, Harano M, Sumitani D, Okajima M. Impact of preoperative skeletal muscle mass and quality on the survival of elderly patients after curative resection of colorectal cancer. J Anus Rectum Colon. 2019;3(4):143–51.
Okumura S, Kaido T, Hamaguchi Y, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157(6):1088–98.
Waki Y, Irino T, Makuuchi R, et al. Impact of preoperative skeletal muscle quality measurement on long-term survival after curative gastrectomy for locally advanced gastric cancer. World J Surg. 2019;43(12):3083–93.
Okumura S, Kaido T, Hamaguchi Y, et al. Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery. 2016;159(3):821–33.
James D. Brierley (Editor) MKGE, Christian Wittekind (Editor). TNM Classification of Malignant Tumours, 8th Edition. Oxford: Wiley-Blackwell; 2017.
Hagi T, Makino T, Yamasaki M, et al. Pathological regression of lymph nodes better predicts long-term survival in esophageal cancer patients undergoing neoadjuvant chemotherapy followed by surgery. Ann Surg. 2020. https://doi.org/10.1097/SLA.0000000000004238.
Makino T, Yamasaki M, Miyazaki Y, et al. Utility of initial induction chemotherapy with 5-fluorouracil, cisplatin, and docetaxel (DCF) for T4 esophageal cancer: a propensity score-matched analysis. Dis Esophagus. 2018;31(4).
Makino T, Yamasaki M, Takemasa I, et al. Dickkopf-1 expression as a marker for predicting clinical outcome in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16(7):2058–64.
Makino T, Yamasaki M, Takeno A, et al. Cytokeratins 18 and 8 are poor prognostic markers in patients with squamous cell carcinoma of the oesophagus. Br J Cancer. 2009;101(8):1298–306.
Hashimoto T, Makino T, Yamasaki M, et al. The pattern of residual tumor after neoadjuvant chemotherapy for locally advanced esophageal cancer and its clinical significance. Ann Surg. 2020;271(5):875–84.
Makino T, Yamasaki M, Tanaka K, et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg. 2019;270(6):1090–5.
Urakawa S, Makino T, Yamasaki M, et al. Lymph node response to neoadjuvant chemotherapy as an independent prognostic factor in metastatic esophageal cancer. Ann Surg. 2019. https://doi.org/10.1097/SLA.0000000000003445.
Yamasaki M, Yasuda T, Yano M, et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann Oncol. 2017;28(1):116–20.
Makino T, Yamasaki M, Miyata H, et al. Solitary lymph node recurrence of esophageal squamous cell carcinoma: surgical failure or systemic disease? Ann Surg Oncol. 2016;23(6):2087–93.
Makino T, Yamasaki M, Tanaka K, et al. Importance of positron emission tomography for assessing the response of primary and metastatic lesions to induction treatments in T4 esophageal cancer. Surgery. 2017;162(4):836–45.
Hagi T, Makino T, Yamasaki M, et al. Dysphagia score as a predictor of adverse events due to triplet chemotherapy and oncological outcomes in 434 consecutive patients with esophageal cancer. Ann Surg Oncol. 2019;26(13):4754–64.
Nc. I. Common Terminology Criteria for Adverse Events v4.0. NIH-publication. 2009;09-7473.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Makino T, Doki Y, Miyata H, et al. Use of (18)F-fluorodeoxyglucose-positron emission tomography to evaluate responses to neo-adjuvant chemotherapy for primary tumor and lymph node metastasis in esophageal squamous cell carcinoma. Surgery. 2008;144(5):793–802.
Makino T, Miyata H, Yamasaki M, et al. Utility of response evaluation to neo-adjuvant chemotherapy by (18)F-fluorodeoxyglucose-positron emission tomography in locally advanced esophageal squamous cell carcinoma. Surgery. 2010;148(5):908–18.
Hamaguchi Y, Kaido T, Okumura S, et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation. 2017;101(3):565–74.
Hamaguchi Y, Kaido T, Okumura S, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32(11–12):1200–5.
Toiyama Y, Miki C, Inoue Y, Tanaka K, Mohri Y, Kusunoki M. Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Exp Ther Med. 2011;2(1):95–101.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–5.
Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–30.
Nishigori T, Okabe H, Tanaka E, Tsunoda S, Hisamori S, Sakai Y. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J Surg Oncol. 2016;113(6):678–84.
Murimwa GZ, Venkat PS, Jin W, et al. Impact of sarcopenia on outcomes of locally advanced esophageal cancer patients treated with neoadjuvant chemoradiation followed by surgery. J Gastrointest Oncol. 2017;8(5):808–15.
Bahat G, Tufan A, Ozkaya H, et al. Relation between hand grip strength, respiratory muscle strength and spirometric measures in male nursing home residents. Aging Male. 2014;17(3):136–40.
Wakabayashi H, Sakuma K. Rehabilitation nutrition for sarcopenia with disability: a combination of both rehabilitation and nutrition care management. J Cachexia Sarcopenia Muscle. 2014;5(4):269–77.
Shirai H, Kaido T, Hamaguchi Y, et al. Preoperative low muscle mass and low muscle quality negatively impact on pulmonary function in patients undergoing hepatectomy for hepatocellular carcinoma. Liver Cancer. 2018;7(1):76–89.
Zoico E, Corzato F, Bambace C, et al. Myosteatosis and myofibrosis: relationship with aging, inflammation and insulin resistance. Arch Gerontol Geriatr. 2013;57(3):411–6.
Wu PY, Huang KS, Chen KM, Chou CP, Tu YK. Exercise, nutrition, and combined exercise and nutrition in older adults with sarcopenia: a systematic review and network meta-analysis. Maturitas. 2021;145:38–48.
Zimmer P, Trebing S, Timmers-Trebing U, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Supportive Care Cancer. 2018;26(2):615–24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
The Human Ethics Review Committee of Osaka University Graduate School of Medicine approved the protocol for this retrospective study, and each participant provided signed consent. All procedures were in accordance with the Declaration of Helsinki.
Consent for Publication
Consent for publication has been obtained from individuals whose data are included in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2021_10025_MOESM3_ESM.tiff
Supplementary file3. Figure S1:Overall and recurrence free survival in patients treated foresophageal carcinoma, classified by either the PMI or the IMAC value. Red lines indicatepatients with normal values. Blue lines indicate patients with low PMI or high IMAC values.(A, D O verall survival , B, E ) recurrence free survival , and (C,F) cancer specific survivalPMI: psoas muscle index; IMAC: intramuscular adipose tissue content (TIFF 32221 kb)
Rights and permissions
About this article
Cite this article
Ishida, T., Makino, T., Yamasaki, M. et al. Quantity and Quality of Skeletal Muscle as an Important Predictor of Clinical Outcomes in Patients with Esophageal Cancer Undergoing Esophagectomy after Neoadjuvant Chemotherapy. Ann Surg Oncol 28, 7185–7195 (2021). https://doi.org/10.1245/s10434-021-10025-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10025-x