Abstract
Background
Local recurrence following resection of retroperitoneal liposarcoma (RLPS) is common. Well-differentiated (WD) and dedifferentiated (DD) RLPS are distinct entities with differing outcomes. A few reports suggest that WDLPS can recur as DDLPS and that DDLPS can recur as WDLPS. This study evaluates whether this change in differentiation from the primary tumor to the first local recurrence impacts long-term outcomes.
Methods
Retrospective review from 22 sarcoma centers identified consecutive patients who underwent resection for a first locally recurrent RLPS from January 2002 to December 2011. Outcomes measured included overall survival, local recurrence, and distant metastasis.
Results
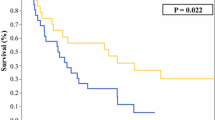
A total of 421 RPLS patients were identified. Of the 230 patients with primary DDLPS, 34 (15%) presented WDLPS upon recurrence (DD → WD); and of the 191 patients with primary WDLPS, 54 (28%) presented DDLPS upon recurrence (WD → DD). The 6-year overall survival probabilities (95% CI) for DD → DD, DD → WD, WD → WD, and WD → DD were 40% (32–48%), 73% (58–92%), 76% (68–85%), and 56% (43–73%) (p < 0.001), respectively. The 6-year second local recurrence incidence was 66% (59–73%), 63% (48–82%), 66% (57–76%), and 77% (66–90%), respectively. The 6-year distant metastasis incidence was 13% (9–19%), 3% (0.4–22%), 5% (2–11%), and 4% (1–16%), respectively. On multivariable analysis, DD → WD was associated with improved overall survival when compared with DD → DD (p < 0.001). Moreover, WD → DD was associated with a higher risk of LR (p = 0.025)
Conclusion
A change in RLPS differentiation from primary tumor to first local recurrence appears to impact survival. These findings may be useful in counseling patients on their prognosis and subsequent management.



Similar content being viewed by others
Change history
19 March 2022
A Correction to this paper has been published: https://doi.org/10.1245/s10434-022-11600-6
References
Bagaria SP, Gabriel E, Mann GN. Multiply recurrent retroperitoneal liposarcoma. J Surg Oncol. 2018;117:62–8.
Gronchi A, Strauss DC, Miceli R. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg. 2016;263(5):1002–9.
Linehan DC, Lewis JJ, Leung D, Brennan MF. Influence of biologic factors and anatomic site in completely resected liposarcoma. J Clin Oncol. 2000;18:1637–43.
Park JO, Qin LX, Prete FP, Antonescu C, Brennan MF, Singer S. Predicting outcome by growth rate of locally recurrent retroperitoneal liposarcoma: the one centimeter per month rule. Ann Surg. 2009;250:977–82.
Hamilton TD, Cannell AJ, Kim M, et al. Results of resection for recurrent or residual retroperitoneal sarcoma after failed primary treatment. Ann Surg Oncol. 2017;24:211–8.
Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–370. discussion 70-1.
Tseng WW, Madewell JE, Wei W, et al. Locoregional disease patterns in well-differentiated and dedifferentiated retroperitoneal liposarcoma: implications for the extent of resection? Ann Surg Oncol. 2014;21:2136–43.
Tan MC, Brennan MF, Kuk D, et al. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surg. 2016;263:593–600.
Lahat G, Anaya DA, Wang X, Tuvin D, Lev D, Pollock RE. Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches. Ann Surg Oncol. 2008;15:1585–93.
Dei Tos AP. Liposarcomas: diagnostic pitfalls and new insights. Histopathology. 2014;64:38–52.
Chan RP, Callegaro D, Miceli R, et al. Predicting survival in patients undergoing resection for locally recurrent retroperitoneal sarcoma: a study and novel nomogram from TARPSWG. Clin Cancer Res. 2019;25(8):2664–71.
Agresti Alan. Categorical data analysis. New York: Wiley; 1990. p. 350–4.
Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37(3/4):256–66.
MacNeill AJ, Gronchi A, Micelli R, et al. Postoperative morbidity after radical resection of primary retroperitoneal sarcoma a report from the transatlantic RPS working group. Ann Surg Oncol. 2017;24:688–9.
Schemper M, Smith TL. A note on quantifying follow-up studies of failure time. Control Clin Trials. 1996;17:343–6.
Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61.
Evans HL. Liposarcoma a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3:507–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
P.R.: honoraria for lectures and Advisory Boards from Novartis, BMS, MSD, Pierre Fabre, Sanfoi, Merck, Pfizer, and Blueprint Medicines. N.A.: consultant for Cepheid; grant funding from Van Andel Research Institute – Stand Up To Cancer Epigenetics Dream Team. Stand Up To Cancer is a program of the Entertainment Industry Foundation, administered by AACR; she is a member of the Scientific Advisory Council to the No Stomach for Cancer Foundation, and sits on the Yale New Haven Hospital Board of Trustees; licensed methylation biomarkers to Cepheid (Patent No. 10167513); shares in Coca-Cola and IBM; her spouse is an IBM employee, and owns a private technology company. G.G.; grants from: Bayer, Pharmamar, Novartis. Personal fees: Novartis, Bayer, Pharmamar, Lilly, EISAI, Merck, and Tesaro. This paper was accepted as an Oral Presentation at the Society of Surgical Oncology Annual Cancer Symposium, Virtual August 17–18, 2020 and voted amongst the Best of SSO 2020.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Elisabetta Pennacchioli’s affiliation was corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nessim, C., Raut, C.P., Callegaro, D. et al. Analysis of Differentiation Changes and Outcomes at Time of First Recurrence of Retroperitoneal Liposarcoma by Transatlantic Australasian Retroperitoneal Sarcoma Working Group (TARPSWG). Ann Surg Oncol 28, 7854–7863 (2021). https://doi.org/10.1245/s10434-021-10024-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10024-y