Abstract
Background
How obesity has an impact on operative and oncologic outcomes for gastric cancer patients is unclear, and the influence of obesity on response to neoadjuvant chemotherapy (NAC) has not been evaluated.
Methods
Patients who underwent curative gastrectomy for primary gastric cancer between 2000 and 2018 were retrospectively identified. After stratification for NAC, operative morbidity, mortality, overall survival (OS), and disease-specific survival (DSS) were compared among three body mass index (BMI) categories: normal BMI (< 25 kg/m2), mild obesity (25–35 kg/m2), and severe obesity (≥ 35 kg/m2).
Results
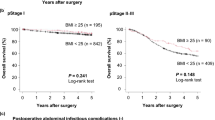
During the study period, 984 patients underwent upfront surgery, and 484 patients received NAC. Tumor stage did not differ among the BMI groups. However, the rates of pathologic response to NAC were significantly lower for the patients with severe obesity (10% vs 40%; p < 0.001). Overall complications were more frequent among the obese patients (44.3% for obese vs 24.9% for normal BMI, p < 0.001). Intraabdominal infections were also more frequent in obese patients (13.9% for obese vs 4.7% for normal BMI, p = 0.001). In the upfront surgery cohort, according to the BMI, OS and DSS did not differ, whereas in the NAC cohort, severe obesity was independently associated with worse OS [hazard ratio (HR) 1.87; 95% confidence interval (CI) 1.01–3.48; p = 0.047] and disease-specific survival (DSS) (HR 2.08; 95% CI 1.07–4.05; p = 0.031).
Conclusion
For the gastric cancer patients undergoing curative gastrectomy, obesity was associated with significantly lower rates of pathologic response to NAC and more postoperative complications, as well as shorter OS and DSS for the patients receiving NAC.



Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–57.
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21.
Calle EE, Thun MJ, Petrelli JM, et al. Body mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–105.
Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38.
Kim MG, Yook JH, Kim KC, et al. Influence of obesity on early surgical outcomes of laparoscopic-assisted gastrectomy in gastric cancer. Surg Laparosc Endosc Percutan Tech. 2011;21:151–4.
Bickenbach KA, Denton B, Gonen M, et al. Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Ann Surg Oncol. 2013;20:780–7.
Pata G, Solaini L, Roncali S, et al. Impact of obesity on early surgical and oncologic outcomes after total gastrectomy with “over-D1” lymphadenectomy for gastric cancer. World J Surg. 2013;37:1072–81.
Wada T, Kunisaki C, Ono HA, et al. Implications of BMI for the prognosis of gastric cancer among the Japanese population. Dig Surg. 2015;32:480–6.
Shimada S, Sawada N, Ishiyama Y, et al. Impact of obesity on short- and long-term outcomes of laparoscopy-assisted distal gastrectomy for gastric cancer. Surg Endosc. 2018;32:358–66.
Kambara Y, Yuasa N, Takeuchi E, et al. Overweight or obesity is an unfavorable long-term prognostic factor for patients who underwent gastrectomy for stage II/III gastric cancer. World J Surg. 2019;43:1766–76.
Sahakyan MA, Shahbazyan SS, Martirosyan A, et al. Gastrectomy for gastric cancer in patients with BMI ≥30 kg/m2. Am Surg. 2020;86:158–63.
Moriwaki Y, Kunisaki C, Kobayashi S, et al. Does body mass index (BMI) influence morbidity and long-term survival in gastric cancer patients after gastrectomy? Hepatogastroenterology. 2003;50:284–8.
Lianos GD, Bali CD, Glantzounis GK, et al. BMI and lymph node ratio may predict clinical outcomes of gastric cancer. Future Oncol. 2014;10:249–55.
Voglino C, Di Mare G, Ferrara F, et al. Clinical and oncological value of preoperative BMI in gastric cancer patients: a single-center experience. Gastroenterol Res Pract. 2015;2015:810134.
Karatas F, Erdem GU, Sahin S, et al. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast. 2017;32:237–44.
Litton JK, Gonzalez-Angulo AM, Warneke CL, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol. 2008;26:4072–7.
Del Fabbro E, Parsons H, Warneke CL, et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist. 2012;17:1240–5.
Amin MB, Greene FL, Edge SB, et al. The eighth-edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–9.
Japanese Gastric Cancer A. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Becker K, Langer R, Reim D, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;253:934–9.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Sugimoto M, Kinoshita T, Shibasaki H, et al. Short-term outcome of total laparoscopic distal gastrectomy for overweight and obese patients with gastric cancer. Surg Endosc. 2013;27:4291–6.
Li SS, Udelsman BV, Parikh A, et al. Impact of postoperative complication and completion of multimodality therapy on survival in patients undergoing gastrectomy for advanced gastric cancer. J Am Coll Surg. 2020;230:912–24.
Saunders JH, Yanni F, Dorrington MS, et al. Impact of postoperative complications on disease recurrence and long-term survival following oesophagogastric cancer resection. Br J Surg. 2020;107:103–12.
Tokunaga M, Kurokawa Y, Machida R, et al. Impact of postoperative complications on survival outcomes in patients with gastric cancer: exploratory analysis of a randomized controlled JCOG1001 trial. Gastric Cancer. 2020;24(1):214–23.
Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol. 2010;17:1159–67.
Tomasello G, Petrelli F, Ghidini M, et al. Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: a meta-analysis of 17 published studies. Eur J Surg Oncol. 2017;43:1607–16.
Smyth EC, Fassan M, Cunningham D, et al. Effect of pathologic tumor response and nodal status on survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol. 2016;34:2721–7.
Xu X, Zheng G, Zhang T, et al. Is pathologic tumor regression grade after neoadjuvant chemotherapy a promising prognostic indicator for patients with locally advanced gastric cancer? A cohort study evaluating tumor regression response. Cancer Chemother Pharmacol. 2019;84:635–46.
Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21:4524–31.
Zahreddine H, Borden KL. Mechanisms and insights into drug resistance in cancer. Front Pharmacol. 2013;4:28.
de Visser KE, Jonkers J. Towards understanding the role of cancer-associated inflammation in chemoresistance. Curr Pharm Des. 2009;15:1844–53.
Mentoor I, Engelbrecht AM, van Jaarsveld PJ, et al. Chemoresistance: intricate interplay between breast tumor cells and adipocytes in the tumor microenvironment. Front Endocrinol Lausanne. 2018;9:758.
Iwase T, Sangai T, Nagashima T, et al. Impact of body fat distribution on neoadjuvant chemotherapy outcomes in advanced breast cancer patients. Cancer Med. 2016;5:41–8.
McQuade JL, Daniel CR, Hess KR, et al. Association of body mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–22.
Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–71.
Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–26.
Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013.
Bang YJ, Van Cutsem E, Fuchs CS, et al. KEYNOTE-585: phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019;15:943–52.
Acknowledgements
This research was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748. We acknowledge Jessica Moore, MS, staff editor at MSK, for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Yelena Y. Janjigian has received research funding from Rgenix, Boehringer Ingelheim, Bayer, Genentech/Roche, Bristol-Myers Squibb, Eli Lilly, and Merck and served on advisory boards for Rgenix, Merck Serono, Bristol-Myers Squibb, Eli Lilly, Pfizer, Bayer, Imugene, Merck, Daiichi-Sankyo, and AstraZeneca. Geoffrey Y. Ku has received honoraria and research funding from Merck, Bristol-Myers Squibb, and Pieris, and research funding from AstraZeneca, Zymeworks, and Daiichi Sankyo. David H. Ilson has received research funding from and served on advisory boards for Astellas, Eli Lilly, Pieris, and Taiho, and has served on advisory boards for Astra-Zeneca, Amgen, Bayer, Bristol-Myers Squibb, and Roche. Steven B. Maron has received research funding from Genentech and Guardant Health and has served on advisory boards for Natera and Basileahas. All the disclosed funding was provided to the institution for other studies. All the other authors declare that they have no financial relationships to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakauchi, M., Vos, E.L., Tang, L.H. et al. Association of Obesity with Worse Operative and Oncologic Outcomes for Patients Undergoing Gastric Cancer Resection. Ann Surg Oncol 28, 7040–7050 (2021). https://doi.org/10.1245/s10434-021-09880-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09880-5