Abstract
Background
This study sought to determine prognostic markers for disease recurrence and survival in a cohort of neoadjuvant-treated, node-negative gastric cancer patients (ypT0-4N0M0).
Methods
Clinicopathologic data from patients treated with neoadjuvant therapy followed by curative-intent gastrectomy at the University of Texas MD Anderson Cancer Center from 1995 to 2017 were evaluated. Patients with AJCC TNM stage ypT0-4N0M0 were considered for analysis.
Results
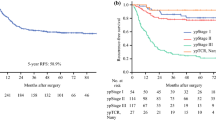
The inclusion criteria were met by 212 patients with a mean age of 58.3 years. Of these patients, 60 % were male, 53 % were Caucasian, 87 % received chemoradiation, and 13 % received chemotherapy. The findings showed a median overall survival (OS) rate of 11.3 years, a 5-year survival rate of 72 %, and a 10-year survival rate of 57 %. During a median follow-up period of 5.5 years, 38.2 % of the patients died. In the multivariable analysis, ypT4-stage and nodal yield fewer than 16 were significantly associated with reduced OS. Cancer classified as ypT4 had more aggressive biologic traits, including lymphovascular and perineural invasion, and was treated more aggressively with total gastrectomy and additional organ resection despite frequent positive margins. Depth of invasion remained significantly associated with worse outcome after the analysis controlled for nodal yield and possible stage migration. Compared with ypT0-3 tumors, ypT4 cancers were associated with significantly more recurrences (13 % vs. 45 %; p < 0.05), and the primary modes of failure for ypT4 lesions were local recurrence and peritoneal metastases (88 % of recurrences).
Conclusions
Depth of primary tumor invasion and nodal yield were significantly associated with OS among the patients with ypT0-4N0M0 gastric cancer. Serosal invasion (ypT4) was associated with a high rate of peritoneal recurrence, and trials of intraperitoneal therapy targeting these patients should be considered.


Similar content being viewed by others
References
Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230–6.
Cancer Stat Facts: Stomach Cancer. National Cancer Institute, 2020. Retrieved 18 August 2020 at https://seer.cancer.gov/statfacts/html/stomach.html.).
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30.
Stark AP, Estrella JS, Chiang YJ, et al. Impact of tumor regression grade on recurrence after preoperative chemoradiation and gastrectomy for gastric cancer. J Surg Oncol. 2020;122:422–32.
Badgwell B, Blum M, Estrella J, et al. Predictors of survival in patients with resectable gastric cancer treated with preoperative chemoradiation therapy and gastrectomy. J Am Coll Surg. 2015;221:83–90.
Ikoma N, Cormier JN, Feig B, et al. Racial disparities in preoperative chemotherapy use in gastric cancer patients in the United States: analysis of the National Cancer Data Base, 2006–2014. Cancer. 2018;124:998–1007.
Ikoma N, Blum M, Estrella JS, et al. Evaluation of the American Joint Committee on Cancer 8th edition staging system for gastric cancer patients after preoperative therapy. Gastric Cancer. 2018;21:74–83.
Ikoma N, Hofstetter WL, Estrella JS, et al. The ypT category does not impact overall survival in node negative gastric cancer. J Surg Oncol. 2018;117:1721–8.
Zhou Y, Yu F, Wu L, et al. Survival after gastrectomy in node-negative gastric cancer: a review and meta-analysis of prognostic factors. Med Sci Monit. 2015;21:1911–9.
Zhao B, Huang X, Zhang J, et al. Clinicopathologic factors associated with recurrence and long-term survival in node-negative advanced gastric cancer patients. Rev Esp Enferm Dig. 2019;111:111–20.
Dittmar Y, Schule S, Koch A, et al. Predictive factors for survival and recurrence rate in patients with node-negative gastric cancer: a European single-centre experience. Langenbecks Arch Surg. 2015;400:27–35.
Jeong JY, Kim MG, Ha TK, Kwon SJ. Prognostic factors on overall survival in lymph node negative gastric cancer patients who underwent curative resection. J Gastric Cancer. 2012;12:210–6.
Lee IS, Yook JH, Kim TH, et al. Prognostic factors and recurrence pattern in node-negative advanced gastric cancer. Eur J Surg Oncol. 2013;39:136–40.
Liu X, Cai H, Shi Y, Wang Y. Prognostic factors in patients with node-negative gastric cancer: a single-center experience from China. J Gastrointest Surg. 2012;16:1123–7.
Seshadri RA, Jayanand SB, Ranganathan R. Prognostic factors in patients with node-negative gastric cancer: an Indian experience. World J Surg Oncol. 2011;9:48.
Saito H, Kuroda H, Matsunaga T, et al. Prognostic indicators in node-negative advanced gastric cancer patients. J Surg Oncol. 2010;101:622–5.
Baiocchi GL, Tiberio GA, Minicozzi AM, et al. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg. 2010;252:70–3.
Huang CM, Wang HM, Zheng CH, et al. Tumor size as a prognostic factor in patients with node-negative gastric cancer invading the muscularis propria and subserosa (pT2-3N0M0 stage). Hepatogastroenterology. 2013;60:699–703.
Chou HH, Kuo CJ, Hsu JT, et al. Clinicopathologic study of node-negative advanced gastric cancer and analysis of factors predicting its recurrence and prognosis. Am J Surg. 2013;205:623–30.
Jin LX, Fields RC. Survival in lymph node-negative gastric cancer: the Western experience. Transl Gastroenterol Hepatol. 2016;1:60.
Kooby DA, Suriawinata A, Klimstra DS, et al. Biologic predictors of survival in node-negative gastric cancer. Ann Surg. 2003;237:828–35; discussion 35–7.
Kim DY, Seo KW, Joo JK, et al. Prognostic factors in patients with node-negative gastric carcinoma: a comparison with node-positive gastric carcinoma. World J Gastroenterol. 2006;12:1182–6.
D’Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–16.
Ikoma N, Chen HC, Wang X, et al. Patterns of initial recurrence in gastric adenocarcinoma in the era of preoperative therapy. Ann Surg Oncol. 2017;24:2679–87.
Sautner T, Hofbauer F, Depisch D, et al. Adjuvant intraperitoneal cisplatin chemotherapy does not improve long-term survival after surgery for advanced gastric cancer. J Clin Oncol. 1994;12:970–4.
Rosen HR, Jatzko G, Repse S, et al. Adjuvant intraperitoneal chemotherapy with carbon-adsorbed mitomycin in patients with gastric cancer: results of a randomized multicenter trial of the Austrian Working Group for Surgical Oncology. J Clin Oncol. 1998;16:2733–8.
Yan TD, Black D, Sugarbaker PH, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14:2702–13.
Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer: meta-analysis of randomized trials. Eur J Surg Oncol. 2014;40:12–26.
Reutovich MY, Krasko OV, Sukonko OG. Hyperthermic intraperitoneal chemotherapy in serosa-invasive gastric cancer patients. Eur J Surg Oncol. 2019;45:2405–11.
Beeharry MK, Zhu ZL, Liu WT, et al. Correction to: Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: personal experience from a randomized case control study. BMC Cancer. 2019;19:1256.
Yamaguchi H, Kitayama J, Ishigami H, et al. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119:3354–8.
Glehen O, Passot G, Villeneuve L, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer. 2014;14:183.
Badgwell B, Blum M, Das P, et al. Phase II Trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann Surg Oncol. 2017;24:3338–44.
Li B, Li Y, Wang W, et al. Incorporation of N0 stage with insufficient numbers of lymph nodes into N1 stage in the seventh edition of the TNM classification improves prediction of prognosis in gastric cancer: results of a single-institution study of 1258 Chinese patients. Ann Surg Oncol. 2016;23:142–8.
Deng J, Yamashita H, Seto Y, Liang H. Increasing the number of examined lymph nodes is a prerequisite for improvement in the accurate evaluation of overall survival of node-negative gastric cancer patients. Ann Surg Oncol. 2017;24:745–53.
Gu P, Deng J, Wang W, et al. Impact of the number of examined lymph nodes on stage migration in node-negative gastric cancer patients: a Chinese multi-institutional analysis with propensity score-matching. Ann Transl Med. 2020;8:938.
Son T, Hyung WJ, Lee JH, et al. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer. 2012;118:4687–93.
Mirkin KA, Hollenbeak CS, Wong J. Greater lymph node retrieval improves survival in node-negative resected gastric cancer in the United States. J Gastric Cancer. 2017;17:306–18.
Deng J, Liu J, Wang W, et al. Validation of clinical significance of examined lymph node count for accurate prognostic evaluation of gastric cancer for the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system. Chin J Cancer Res. 2018;30:477–91.
Acknowledgement
Editorial support was provided by Bryan Tutt, Scientific Editor, Research Medical Library.
Author information
Authors and Affiliations
Contributions
DJE performed data acquisition, data analysis and interpretation, and manuscript construction. MB, JSE, PD, BDM, JAA, PFM, BDB, and NI contributed to data acquisition and analysis, provided expert clinical opinion, and contributed to manuscript creation.
Corresponding author
Ethics declarations
Disclosures
PD: Honorarium- Adlai Nortye, Honorarium - ASTRO, Honorarium - Leidos/ NCI.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Erstad, D.J., Blum, M., Estrella, J.S. et al. Determinants of Survival for Patients with Neoadjuvant-Treated Node-Negative Gastric Cancer. Ann Surg Oncol 28, 6638–6648 (2021). https://doi.org/10.1245/s10434-021-09625-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09625-4