Abstract
Background
Adjuvant radiation therapy (RT) can decrease lymph node basin (LNB) recurrences in patients with clinically evident melanoma lymph node (LN) metastases following lymphadenectomy, but its role in the era of modern systemic therapies (ST), immune checkpoint or BRAF/MEK inhibitors, is unclear.
Patients and Methods
Patients at four institutions who underwent lymphadenectomy (1/1/2010–12/31/2019) for clinically evident melanoma LN metastases and received neoadjuvant and/or adjuvant ST with RT, or ST alone, but met indications for RT, were identified. Comparisons were made between ST alone and ST/RT groups. The primary outcome was 3-year cumulative incidence (CI) of LNB recurrence. Secondary outcomes included 3-year incidences of in-transit/distant recurrence and survival estimates.
Results
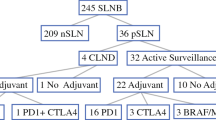
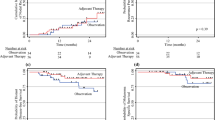
Of 98 patients, 76 received ST alone and 22 received ST/RT. Median follow-up time for patients alive at last follow-up was 44.6 months. The ST/RT group had fewer inguinal node metastases (ST 36.8% versus ST/RT 9.1%; P = 0.04), and more extranodal extension (ST 50% versus ST/RT 77.3%; P = 0.02) and positive lymphadenectomy margins (ST 2.6% versus ST/RT 13.6%; P = 0.04). The 3-year CI of LNB recurrences was lower for the ST/RT group compared with the ST group (13.9% versus 25.2%), but this reduction was not statistically significant (P = 0.36). Groups did not differ significantly in in-transit/distant recurrences (P = 0.24), disease-free survival (P = 0.14), or melanoma-specific survival (P = 0.20).
Conclusions
In the era of modern ST, RT may still have value in reducing LNB recurrences in melanoma with clinical LN metastases. Further research should focus on whether select patient populations derive benefit from combination therapy, and optimizing indications for RT following neoadjuvant ST.





Similar content being viewed by others
References
Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–92. https://doi.org/10.3322/caac.21409
Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–9. https://doi.org/10.1200/jco.2009.27.1627
Lee RJ, Gibbs JF, Proulx GM, Kollmorgen DR, Jia C, Kraybill WG. Nodal basin recurrence following lymph node dissection for melanoma: implications for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. Jan 2000;46(2):467–74. https://doi.org/10.1016/s0360-3016(99)00431-9
Strom T, Torres-Roca JF, Parekh A, et al. Regional radiation therapy impacts outcome for node-positive cutaneous melanoma. J Natl Compr Canc Netw. 2017;15(4):473–82. https://doi.org/10.6004/jnccn.2017.0047
Ballo MT, Strom EA, Zagars GK, et al. Adjuvant irradiation for axillary metastases from malignant melanoma. Int J Radiat Oncol Biol Phys. 2002;52(4):964–72. https://doi.org/10.1016/s0360-3016(01)02742-0
Ballo MT, Bonnen MD, Garden AS, et al. Adjuvant irradiation for cervical lymph node metastases from melanoma. Cancer. 2003;97(7):1789–96. https://doi.org/10.1002/cncr.11243
Ballo MT, Zagars GK, Gershenwald JE, et al. A critical assessment of adjuvant radiotherapy for inguinal lymph node metastases from melanoma. Ann Surg Oncol. 2004;11(12):1079–84. https://doi.org/10.1245/aso.2004.12.039
Agrawal S, Kane JM, Guadagnolo BA, Kraybill WG, Ballo MT. The benefits of adjuvant radiation therapy after therapeutic lymphadenectomy for clinically advanced, high-risk, lymph node-metastatic melanoma. Cancer. 2009;115(24):5836–44. https://doi.org/10.1002/cncr.24627
Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13(6):589–97. https://doi.org/10.1016/s1470-2045(12)70138-9
Henderson MA, Burmeister BH, Ainslie J, et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. 2015;16(9):1049–60. https://doi.org/10.1016/s1470-2045(15)00187-4
Wuthrick EJ, Chablani P. Do not forget about the importance of loco-regional therapy in melanoma management. Semin Radiat Oncol. 2019;29(2):166–70. https://doi.org/10.1016/j.semradonc.2018.11.006
Network NCC. Cutaneous Melanoma, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. Accessed September 6, 2020. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–55. https://doi.org/10.1056/nejmoa1611299
Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–35. https://doi.org/10.1056/nejmoa1709030
Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–23. https://doi.org/10.1056/nejmoa1708539
Maio M, Lewis K, Demidov L, et al. Adjuvant vemurafenib in resected, BRAF. Lancet Oncol. 2018;19(4):510–20. https://doi.org/10.1016/s1470-2045(18)30106-2
Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. https://doi.org/10.1056/nejmoa1802357
Song Y, Tieniber AD, Gimotty PA, et al. Survival outcomes of patients with clinical stage III melanoma in the era of novel systemic therapies. Ann Surg Oncol. 2019;26(13):4621–30. https://doi.org/10.1245/s10434-019-07599-y
Tarhini A, Lin Y, Lin H, et al. Neoadjuvant ipilimumab (3 mg/kg or 10 mg/kg) and high dose IFN-α2b in locally/regionally advanced melanoma: safety, efficacy and impact on T-cell repertoire. J Immunother Cancer. 2018;6(1):112. https://doi.org/10.1186/s40425-018-0428-5
Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–61. https://doi.org/10.1038/s41591-019-0357-y
Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–61. https://doi.org/10.1038/s41591-018-0198-0
Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948–60. https://doi.org/10.1016/s1470-2045(19)30151-2
Amaria RN, Prieto PA, Tetzlaff MT, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018;19(2):181–93. https://doi.org/10.1016/s1470-2045(18)30015-9
Long GV, Saw RPM, Lo S, et al. Neoadjuvant dabrafenib combined with trametinib for resectable, stage IIIB-C, BRAF. Lancet Oncol. 2019;20(7):961–71. https://doi.org/10.1016/s1470-2045(19)30331-6
Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–54. https://doi.org/10.1038/s41591-018-0197-1
Mitra D, Bishop A, Guadagnolo BA. Adjuvant nodal radiation therapy for melanoma in the era of immunotherapy. Int J Radiat Oncol Biol Phys. 2020;108(1):164–9. https://doi.org/10.1016/j.ijrobp.2020.06.006
Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–6. https://doi.org/10.1016/0360-3016(95)00060-c
Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301–8. https://doi.org/10.1158/1078-0432.ccr-11-2097
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–6. https://doi.org/10.1016/0197-2456(96)00075-x
StataCorp. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC; 2019.
Barker CA. Adjuvant pembrolizumab in resected stage III melanoma. N Engl J Med. 2018;379(6):593. https://doi.org/10.1056/nejmc1807505
Hauschild A, Dummer R, Schadendorf D, et al. Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected. J Clin Oncol. 2018;36(35):3441–9. https://doi.org/10.1200/jco.18.01219
Kan CE, Mansur DB. The role of radiation therapy in the management of cutaneous melanoma. Melanoma Manag. 2016;3(1):61–72. https://doi.org/10.2217/mmt.15.41
Burmeister BH, Mark Smithers B, Burmeister E, et al. A prospective phase II study of adjuvant postoperative radiation therapy following nodal surgery in malignant melanoma-Trans Tasman Radiation Oncology Group (TROG) Study 96.06. Radiother Oncol. 2006;81(2):136–42. https://doi.org/10.1016/j.radonc.2006.10.001
Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483–93. https://doi.org/10.1200/jco.1997.15.7.2483
Group MRCOCW. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359(9319):1727–33. https://doi.org/10.1016/s0140-6736(02)08651-8
Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–66. https://doi.org/10.1056/nejmoa022148
Versluis JM, Long GV, Blank CU. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat Med. 2020;26(4):475–84. https://doi.org/10.1038/s41591-020-0829-0
Pelster MS, Amaria RN. Neoadjuvant immunotherapy for locally advanced melanoma. Curr Treat Options Oncol. 2020;21(2):10. https://doi.org/10.1007/s11864-020-0700-z
Deshmane V, Kalloli M, Chikaraddi S, Keerthi B, Krishnappa R. Predictive factors for loco regional recurrence and distant metastasis following primary surgical treatment of cutaneous melanoma. Indian J Dermatol. 2014;59(3):241–6. https://doi.org/10.4103/0019-5154.131383
Funding
No external funding was received for this study.
Author information
Authors and Affiliations
Contributions
Authors responsible for statistical analyses: RJS, MD and YS, MD (same as lead authors). Conception and design of the study: Straker, Song, Lukens, Karakousis. Acquisition of data: Straker, Song, Sun, Krause, Cohen, Muradova, Daou, Frederick, Lee. Analysis and interpretation of data: Straker, Song, Shannon, Krause, Boland, Beasley, Sondak, Wuthrick, Zager, Lin, Lukens, Karakousis. Drafting and critically revising the manuscript: Straker, Song, Sun, Shannon, Krause, Cohen, Muradova, Daou, Frederick, Lee, Boland, Beasley, Sondak, Wuthrick, Krause, Boland, Zager, Lin, Lukens, Karakousis.
Corresponding author
Ethics declarations
Disclosure
Dr. Sondak reports personal fees from Array, Bristol Myers Squibb, Genentech, Merck, Novartis, Pfizer, Regeneron, and Replimune, outside the submitted work. Dr. Wuthrick reports personal fees from Varian and Sanofi, outside the submitted work. Dr. Zager reports advisory board participation with Merck, Amgen, and Sanofi Regeneron; speakers bureau participation with Array Biopharma and Sun Pharma; and research work with Amgen, Provectus, Castle Biosciences, and Delcath Systems, all outside the submitted work. Dr. Boland has sponsored research agreements with Palleon Pharmaceuticals and Olink Proteomics. She served on scientific advisory boards for Nektar Therapeutics and Novartis. She has received honoraria from Novartis and Takeda Pharmaceutics. She served as a consultant to Northwest Biotherapeutics. Dr. Georgia Beasley reports relevant financial activities outside the submitted work as a member of the 2020 Regeneron Sanofi advisory board. Other authors declare no conflicts of interest.
Ethical Approval
All authors have reviewed and approved the submitted manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Straker, R.J., Song, Y., Sun, J. et al. Adjuvant Radiation Therapy for Clinical Stage III Melanoma in the Modern Therapeutic Era. Ann Surg Oncol 28, 3512–3521 (2021). https://doi.org/10.1245/s10434-020-09384-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09384-8