Abstract
Background
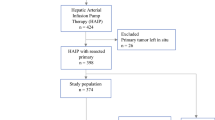
Hepatic artery infusion pump (HAIP) chemotherapy is an advanced cancer therapy for primary and secondary hepatic malignancies. The risk of concurrent hepatic and/or colorectal operations with HAIP placement is unknown. Our objective was to characterize the short-term outcomes of concurrent surgery with HAIP placement.
Methods
The 2005–2017 ACS NSQIP dataset was queried for patients undergoing hepatic and colorectal operations with or without HAIP placement. Outcomes were compared for HAIP placement with different combined procedures. Patients who underwent procedures without HAIP placement were propensity score matched with those with HAIP placement. The primary outcome was 30-day death or serious morbidity (DSM). Secondary outcomes included infectious complications, wound complications, length of stay (LOS), and operative time.
Results
Of 467 patients who underwent HAIP placement, 83.9% had concurrent surgery. The rate of DSM was 10.7% for HAIP placement alone, 19.2% with concurrent minor hepatic procedures, 22.1% with concurrent colorectal resection, 23.2% with concurrent minor hepatic plus colorectal procedures, 28.4% with concurrent major hepatic resection, and 41.7% with concurrent major hepatic plus colorectal resection. On matched analyses, there was no difference in DSM, infectious, or wound complications for procedures with HAIP placement compared with the additional procedure alone, but operative time (294.7 vs 239.8 min, difference 54.9, 95% CI 42.8–67.0) and LOS (6 vs 5, IRR 1.20, 95% CI 1.08–1.33) were increased.
Conclusions
HAIP placement is not associated with additional morbidity when performed with hepatic and/or colorectal surgery. Decisions regarding HAIP placement should consider the risks of concurrent operations, and patient- and disease-specific factors.
Similar content being viewed by others
References
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Colon Cancer (Version 1.2020) https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed February 11, 2020.
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Hepatobiliary Cancers (Version 4.2019). https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed February 11, 2020.
Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol. 2003;14 Suppl 2:ii13-16.
Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657-1669.
Datta J, Narayan RR, Kemeny NE, D’Angelica MI. Role of hepatic artery infusion chemotherapy in treatment of initially unresectable colorectal liver metastases: a review. JAMA Surg. 2019;154(8):768–776.
Schlick CJR, Merkow RP, Bentrem DJ. Nonresectional regional therapies for metastatic colorectal cancer to the liver. J Surg Oncol. 2019;119(5):636-641.
Karanicolas PJ, Metrakos P, Chan K, et al. Hepatic arterial infusion pump chemotherapy in the management of colorectal liver metastases: expert consensus statement. Curr Oncol. 2014;21(1):e129-136.
Ko YJ, Karanicolas PJ. Hepatic arterial infusion pump chemotherapy for colorectal liver metastases: an old technology in a new era. Curr Oncol. 2014;21(1):e116-121.
Ellis RJ, Angelos P, Jarnagin WR, Kemeny NE, Merkow RP. Abrupt Discontinuation of the Codman Hepatic Artery Infusion Pump: Considerations in the Era of Precision Medicine. J Am Coll Surg. 2019;229(2):217-219.
Liver-directed chemotherapy device is saving lives. The Globe and Mail. 2013. https://www.theglobeandmail.com/life/health-and-fitness/advsunnybrook/healing-the-future/liver-directed-chemotherapy-device-is-saving-lives/article12379031/. Accessed February 2020.
Buisman FE, Homs MYV, Grunhagen DJ, et al. Adjuvant hepatic arterial infusion pump chemotherapy and resection versus resection alone in patients with low-risk resectable colorectal liver metastases—the multicenter randomized controlled PUMP trial. BMC Cancer. 2019;19(1):327.
Dizon DS, Schwartz J, Kemeny N. Regional chemotherapy: a focus on hepatic artery infusion for colorectal cancer liver metastases. Surg Oncol Clin N Am. 2008;17(4):759-771, viii.
Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. 2005;201(1):57-65.
Daly JM, Kemeny N, Oderman P, Botet J. Long-term hepatic arterial infusion chemotherapy. Anatomic considerations, operative technique, and treatment morbidity. Arch Surg. 1984;119(8):936-941.
Martin RC, Edwards MJ, McMasters KM. Morbidity of adjuvant hepatic arterial infusion pump chemotherapy in the management of colorectal cancer metastatic to the liver. Am J Surg. 2004;188(6):714-721.
Khuri SF, Daley J, Henderson W, et al. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg. 1998;228(4):491-507.
Khuri SF, Henderson WG, Daley J, et al. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
Khuri SF, Henderson WG, Daley J, et al. The patient safety in surgery study: background, study design, and patient populations. J Am Coll Surg. 2007;204(6):1089-1102.
Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336-346 e331.
Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44:251-267.
National Quality Forum. NQF #0697: Risk Adjusted Case Mix Adjusted Elderly Surgery Outcomes Measure. http://www.qualityforum.org. Published 2017. Accessed 4 May 2020.
National Quality Forum. NQF #0706: Risk Adjusted Colon Surgery Outcome Measure. http://www.qualityforum.org. Published 2017. Accessed 4 May 2020.
Merkow RP, Hall BL, Cohen ME, et al. Validity and feasibility of the American College of Surgeons colectomy composite outcome quality measure. Ann Surg. 2013;257(3):483-489.
Bilimoria KY, Chung JW, Hedges LV, et al. National Cluster-Randomized Trial of Duty-Hour Flexibility in Surgical Training. N Engl J Med. 2016;374(8):713-727.
SAS Institute Inc. SAS/STAT(R) 14.2 User’s Guide. In: Cary, NC: SAS Institute Inc.; 2016.
D’Angelica MI, Correa-Gallego C, Paty PB, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015;261(2):353-360.
Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122(5):758-765.
Lim A, Le Sourd S, Senellart H, et al. Hepatic Arterial Infusion Chemotherapy for Unresectable Liver Metastases of Colorectal Cancer: A Multicenter Retrospective Study. Clin Colorectal Cancer. 2017;16(4):308-315.
Lorenz M, Muller HH, Schramm H, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen). Ann Surg. 1998;228(6):756-762.
Alberts SR, Roh MS, Mahoney MR, et al. Alternating systemic and hepatic artery infusion therapy for resected liver metastases from colorectal cancer: a North Central Cancer Treatment Group (NCCTG)/National Surgical Adjuvant Breast and Bowel Project (NSABP) phase II intergroup trial, N9945/CI-66. J Clin Oncol. 2010;28(5):853-858.
Levi FA, Boige V, Hebbar M, et al. Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Ann Oncol. 2016;27(2):267-274.
Acknowledgments
DJB is supported by the Veteran’s Administration (I01HX002290). ADY is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (K08HL145139). RPM is supported by the Agency for Healthcare Research and Quality (K12HS026385) and an Institutional Research Grant from the American Cancer Society (IRG-18-163-24). DM provides advising or speaking services for Amgen Inc., Bristol Meyers Squibb Company, Exelixis Inc., Eisai Co. Ltd., and Genentech Inc. and receives research funding from Merck & Co. Inc. and Oncolytics Biotech Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
DM provides advising or speaking services for Amgen Inc., Bristol Meyers Squibb Company, Exelixis Inc., Eisai Co. Ltd., and Genentech Inc. and receives research funding from Merck & Co. Inc. and Oncolytics Biotech Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1: Current procedure terminology (CPT) codes included in each cohort
Appendix 1: Current procedure terminology (CPT) codes included in each cohort
Operative cohort | CPT codes |
|---|---|
Hepatic artery infusion pump placement | 36260 |
Colon or rectum resection | 44120, 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45130, 45135, 45160, 45395, 45397, 45402, 45550 |
Major hepatic resection | 47122, 47125, 47130 |
Minor hepatic procedures | 47100, 47120, 47370, 47371, 47379, 47380, 47381, 47382, 47383, 47399 |
Rights and permissions
About this article
Cite this article
Brajcich, B.C., Bentrem, D.J., Yang, A.D. et al. Short-Term Risk of Performing Concurrent Procedures with Hepatic Artery Infusion Pump Placement. Ann Surg Oncol 27, 5098–5106 (2020). https://doi.org/10.1245/s10434-020-08938-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08938-0