Abstract
Background
High-mobility group box-1 (HMGB1) is involved in a broad range of inflammatory responses and the progression of various types of malignancy. However, the roles of HMGB1 in the progression of esophageal squamous cell carcinoma (ESCC) are unclear. The aim of this study was to investigate the significance of intracellular and extracellular HMGB1 in ESCC.
Methods
HMGB1 levels were measured in the tissue and plasma of patients with ESCC, or in ESCC cell lines and their conditioned medium. The effects of downregulation of intracellular HMGB1 or upregulation of extracellular HMGB1 on proliferation, cell migration, and invasion were evaluated using proliferation, transwell, and wound healing assays.
Results
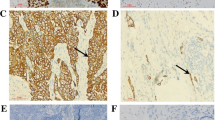
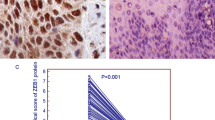
Downregulation of HMGB1 expression inhibited cell proliferation, migration, and invasion. On the other hand, upregulation of extracellular HMGB1 level by addition of recombinant HMGB1 promoted the migratory and invasive abilities of ESCC cells through increases of phosphorylation of the signal-regulated kinase 1/2 and NF-κBp65 proteins. These effects of extracellular HMGB1 were attenuated by treatment with recombinant soluble thrombomodulin, which adsorbs HMGB1. The expression of HMGB1 was significantly higher in tumor tissue (p = 0.008), and the concentration of HMGB1 in the plasma was significantly higher in patients with ESCC than in healthy volunteers (p = 0.04). Cancer-specific survival was worse in patients with high concentration of plasma HMGB1 (p = 0.01).
Conclusion
Increase of HMGB1 levels in tumor cells or plasma plays a crucial role in the malignant potential of ESCC. Intracellular and extracellular HMGB1 may be a therapeutic target in ESCC.




Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015, 136, E359–E386.
Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014, 371, 2499–2509.
Cohen DJ, Leichman L. Controversies in the treatment of local and locally advanced gastric and esophageal cancers. J Clin Oncol. 2015, 33, 1754–1759.
Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973, 38, 14–19.
Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005, 5, 331–342.
Chuangui C, Peng T, Zhentao Y. The expression of high mobility group box 1 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Pathol Oncol Res. 2012, 18, 1021–1027.
Ma H, Zheng S, Zhang X, Gong T, Lv X, Fu S, et al. High mobility group box 1 promotes radioresistance in esophageal squamous cell carcinoma cell lines by modulating autophagy. Cell Death Dis. 2019, 10, 136.
Tang D, Kang R, Zeh HJ 3rd, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010, 1799, 131–140.
Wu T, Zhang W, Yang G, Li H, Chen Q, Song R, et al. HMGB1 overexpression as a prognostic factor for survival in cancer: a meta-analysis and systematic review. Oncotarget. 2016, 7, 50417–50427.
Yao X, Zhao G, Yang H, Hong X, Bie L, Liu G. Overexpression of high-mobility group box 1 correlates with tumor progression and poor prognosis in human colorectal carcinoma. J Cancer Res Clin Oncol. 2010, 136, 677–684.
Chen S, Dong Z, Yang P, Wang X, Jin G, Yu H, et al. Hepatitis B virus X protein stimulates high mobility group box 1 secretion and enhances hepatocellular carcinoma metastasis. Cancer Lett. 2017, 394, 22–32.
Zhang J, Kou YB, Zhu JS, Chen WX, Li S. Knockdown of HMGB1 inhibits growth and invasion of gastric cancer cells through the NF-κB pathway in vitro and in vivo. Int J Oncol. 2014, 44, 1268–1276.
Chung HW, Lee SG, Kim H, Hong DJ, Chung JB, Stroncek D, et al. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J Transl Med. 2009, 7, 38.
Cheng BQ, Jia CQ, Liu CT, Lu XF, Zhong N, Zhang ZL, et al. Serum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Dig Liver Dis. 2008, 40, 446–452.
Shang GH, Jia CQ, Tian H, Xiao W, Li Y, Wang AH, et al. Serum high mobility group box protein 1 as a clinical marker for non-small cell lung cancer. Respir Med. 2009, 103, 1949–1953.
Lee H, Song M, Shin N, Shin CH, Min BS, Kim HS, et al. Diagnostic significance of serum HMGB1 in colorectal carcinomas. PLoS One. 2012, 7, e34318.
Chung HW, Jang S, Kim H, Lim JB. Combined targeting of high-mobility group box-1 and interleukin-8 to control micrometastasis potential in gastric cancer. Int J Cancer. 2015, 137, 1598–1609.
Zhu L, Li X, Chen Y, Fang J, Ge Z. High-mobility group Box 1: a novel inducer of the epithelial–mesenchymal transition in colorectal carcinoma. Cancer Lett. 2015, 357, 527–534.
Candolfi M, Yagiz K, Foulad D, Alzadeh GE, Tesarfreund M, Muhammad AK, et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin Cancer Res. 2009, 15, 4401–4414.
Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000, 405, 354–360.
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010, 28, 367–388.
Schlueter C, Weber H, Meyer B, Rogalla P, Röser K, Hauke S, et al. Angiogenetic signaling through hypoxia, HMGB1, an angiogenetic switch molecule. Am J Pathol. 2005, 166, 1259–1263.
Jube S, Rivera ZS, Bianchi ME, Powers A, Wang E, Pagano I, et al. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012, 72, 3290–3301.
Esmon CT. Coagulation and inflammation. J Endotoxin Res. 2003, 9, 192–198.
Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005, 115, 1267–1274.
Ito T, Kawahara K, Okamoto K, Yamada S, Yasuda M, Imaizumi H, et al. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol. 2008, 28, 1825–1830.
Shirai Y, Uwagawa T, Shiba H, Shimada Y, Horiuchi T, Saito N, et al. Recombinant thrombomodulin suppresses tumor growth of pancreatic cancer by blocking thrombin-induced PAR1 and NF-kB activation. Surgery. 2017, 161, 1675–1682.
Hanly AM, Winter DC. The role of thrombomodulin in malignancy. Semin Thromb Hemost. 2007, 33, 673–679.
Yang Y, Cheng BJ, Lu S. Thrombomodulin regulates doxorubicin sensitivity through epithelial-mesenchymal transition in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2017, 21, 95–101.
Sobin LH, Gospodarowicz MK, Wittekind Ch (eds). TNM classification of malignant tumors, 7th edn. Wiley-Blackwell: Hoboken, 2009.
Das N, Dewan V, Grace PM, Gunn RJ, Tamura R, Tzarum N, et al. HMGB1 activates proinflammatory signaling via TLR5 leading to allodynia. Cell Rep. 2016, 17, 1128–1140.
Zhang L, Chen W, Li X. A novel anticancer effect of butein, inhibition of invasion through the erk1/2 and NF-kappa B signaling pathways in bladder cancer cells. FEBS Lett. 2008, 582, 1821–1828.
Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009, 28, 113–127.
Kao YC, Wu LW, Shi CS, Chu CH, Huang CW, Kuo CP, et al. Downregulation of thrombomodulin, a novel target of Snail, induces tumorigenesis through epithelial-mesenchymal transition. Mol Cell Biol. 2010, 30, 4767–4785.
Wu CT, Chang YH, Lin P, Chen WC, Chen MF. Thrombomodulin expression regulates tumorigenesis in bladder cancer. BMC Cancer. 2014, 14, 375.
van Beijnum JR, Nowak-Sliwinska P, van den Boezem E, Hautvast P, Buurman WA, Griffioen AW. Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene. 2013, 32, 363–374.
Acknowledgments
We wish acknowledge Asahi Kasei Pharma (Tokyo, Japan) for providing the human recombinant soluble thrombomodulin. There was no source of funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsubara, D., Konishi, H., Arita, T. et al. Involvement of Intracellular and Extracellular High-Mobility Group Box-1 in the Progression of Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 27, 3233–3244 (2020). https://doi.org/10.1245/s10434-020-08363-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08363-3