Abstract
Background
Neoadjuvant chemotherapy (NAC) for breast cancer increases breast-conserving surgery (BCS) rates, but many women opt for mastectomy with contralateral prophylactic mastectomy (CPM). Here we evaluate factors associated with CPM use in women undergoing mastectomy post-NAC.
Methods
A retrospective institutional NAC database review identified women with clinical stage I-III, unilateral invasive breast cancer undergoing unilateral mastectomy (UM) or CPM mastectomy from 9/2013 to 12/2017. Clinical/pathologic characteristics, imaging, and presence of contraindications to BCS post-NAC were compared, with subset analysis of BCS candidates. The multivariable analysis was adjusted for potential confounders.
Results
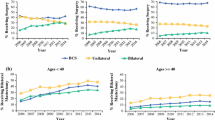
Five hundred sixty-nine women underwent mastectomy after NAC, 297 (52%) UM and 272 (48%) CPM. On univariable analysis, younger age, BRCA+, lower pre-NAC clinical stage, pathologic complete response, and axillary surgery extent were associated with CPM (all p < 0.01). Favorable post-NAC clinical factors of no residual palpable disease, clinically negative nodes, complete response on breast imaging, and no post-NAC contraindication to BCS were also associated with CPM (all p < 0.01). On multivariable analysis, young age (odds ratio [OR] 0.93, 95% confidence interval [CI] 0.91–0.95), lower pre-NAC stage (OR 0.51, 95% CI 0.34–0.77), and no contraindication to BCS (OR 3.12, 95% CI 2.02–4.82) were significantly associated with CPM. Among the 203 (35%) women who had no contraindications to BCS post-NAC, 145 (71%) underwent CPM. BRCA+ and family history were reasons more frequently cited for mastectomy among CPM than UM (p < 0.001).
Conclusions
CPM was performed in 48% of women undergoing mastectomy after NAC; younger women with earlier-stage cancers were more likely to undergo CPM. While increased use of CPM in women with more favorable disease is medically appropriate, our findings indicate a lost opportunity for use of BCS.

Similar content being viewed by others
References
Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007;18(2):Cd005002.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85.
de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15(10):1137–46.
Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30(16):1989-95.
Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg. 2015;262(3):434–9; discussion 8–9.
Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II-III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance). Breast Cancer Res Treat 2016;160(2):297–304.
7. Kantor O, Ajmani G, Wang CH, Datta A, Yao K. The shifting paradigm for breast cancer surgery in patients undergoing neoadjuvant chemotherapy. Ann Surg Oncol. 2018;25(1):164–72.
Pollom EL, Qian Y, Chin AL, et al. Rising rates of bilateral mastectomy with reconstruction following neoadjuvant chemotherapy. Int J Cancer. 2018;143(12):3262–72.
Wapnir IL, Kurian AW, Lichtensztajn DY, Clarke CA, Gomez SL. Rising bilateral mastectomy rates among neoadjuvant chemotherapy recipients in California from 1998 to 2012. Ann Surg. 2017;266(2):353–60.
Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann Surg 2017;265(3):581–9.
Hunt KK, Euhus DM, Boughey JC, et al. Society of Surgical Oncology Breast Disease Working Group statement on prophylactic (risk-reducing) mastectomy. Ann Surg Oncol. 2017;24(2):375–97.
Portschy PR, Kuntz KM, Tuttle TM. Survival outcomes after contralateral prophylactic mastectomy: a decision analysis. J Natl Cancer Inst. 2014;106(8).
Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203–9.
Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362–7.
Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56(4):1038–45.
King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158–64.
Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–46.
Basu NN, Ross GL, Evans DG, Barr L. The Manchester guidelines for contralateral risk-reducing mastectomy. World J Surg Oncol. 2015;13:237.
Bedrosian I, Hu CY, Chang GJ. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J Natl Cancer Inst. 2010;102(6):401–9.
Peralta EA, Ellenhorn JD, Wagman LD, Dagis A, Andersen JS, Chu DZ. Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. Am J Surg 2000;180(6):439–45.
Jagsi R, Hawley ST, Griffith KA, et al. Contralateral prophylactic mastectomy decisions in a population-based sample of patients with early-stage breast cancer. JAMA Surg 2017;152(3):274–82.
Mougalian SS, Soulos PR, Killelea BK, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer. 2015;121(15):2544–52.
Pilewskie M, Zabor EC, Mamtani A, Barrio AV, Stempel M, Morrow M. The optimal treatment plan to avoid axillary lymph node dissection in early-stage breast cancer patients differs by surgical strategy and tumor subtype. Ann Surg Oncol. 2017;24(12):3527–33.
Boughey JC, Attai DJ, Chen SL, et al. Contralateral prophylactic mastectomy (CPM) consensus statement from the American Society of Breast Surgeons: data on CPM outcomes and risks. Ann Surg Oncol. 2016;23(10):3100–5.
25. National Institutes of Health. NIH consensus conference. Treatment of early-stage breast cancer. Jama 1991;265(3):391–5.
Cassidy MR, Zabor EC, Stempel M, Mehrara B, Gemignani ML. Does response to neo-adjuvant chemotherapy impact breast reconstruction? Breast J. 2018;24(4):567–73.
Acknowledgements
This study was presented in poster format at the Society of Surgical Oncology 72nd Annual Cancer Symposium, March 27–30, 2019, San Diego, CA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The preparation of this manuscript was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center, Dr. Monica Morrow has received speaking honoraria from Genomic Health and Roche.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Christian, N., Zabor, E.C., Cassidy, M. et al. Contralateral Prophylactic Mastectomy Use After Neoadjuvant Chemotherapy. Ann Surg Oncol 27, 743–749 (2020). https://doi.org/10.1245/s10434-019-08038-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-08038-8