Abstract
Background
The optimal surgical method for cT1N0 lung adenocarcinoma remains controversial.
Objective
The aim of this study was to evaluate the differences in clinical outcomes of sublobar resection and lobectomy for cT1N0 lung adenocarcinoma patients.
Methods
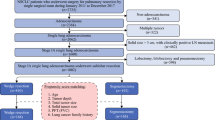
We included 1035 consecutive patients with cT1N0 lung adenocarcinoma who underwent surgery at our institute from January 2011 to December 2016. The surgical approach, either sublobar resection or lobectomy, was determined at the discretion of each surgeon. A propensity-matched analysis incorporating total tumor diameter, solid component diameter, consolidation-to-tumor (C/T) ratio, and performance status was used to compare the clinical outcomes of the sublobar resection and lobectomy groups.
Results
Sublobar resection and lobectomy were performed for 604 (58.4%; wedge resection/segmentectomy: 470/134) and 431 (41.6%) patients, respectively. Patients in the sublobar resection group had smaller total tumor diameters, smaller solid component diameters, lower C/T ratios, and better performance status. More lymph nodes were dissected in the lobectomy group. Patients in the sublobar resection group had better perioperative outcomes. A multivariable analysis revealed that the solid component diameter and serum carcinoembryonic antigen level are independent risk factors for tumor recurrence. After propensity matching, 284 paired patients in each group were included. No differences in overall survival (OS; p = 0.424) or disease-free survival (DFS; p = 0.296) were noted between the two matched groups.
Conclusions
Sublobar resection is not inferior to lobectomy regarding both DFS and OS for cT1N0 lung adenocarcinoma patients. Sublobar resection may be a feasible surgical method for cT1N0 lung adenocarcinoma.


Similar content being viewed by others
References
Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22 (discussion 22-3).
Speicher PJ, Gu L, Gulack BC, et al. Sublobar resection for clinical stage IA non-small-cell lung cancer in the United States. Clin Lung Cancer. 2016;17:47–55.
Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer ≤ 1 cm in size: a review of SEER data. Chest. 2011;139:491–6.
Whitson BA, Groth SS, Andrade RS, et al. Invasive adenocarcinoma with bronchoalveolar features: a population-based evaluation of the extent of resection in bronchoalveolar cell carcinoma. J Thorac Cardiovasc Surg. 2012;143:591–600.
Yendamuri S, Sharma R, Demmy M, et al. Temporal trends in outcomes following sublobar and lobar resections for small (≤ 2 cm) non-small cell lung cancers: a surveillance epidemiology end results database analysis. J Surg Res. 2013;183:27–32.
Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg. 2005;80:2041–5.
McMurry TL, Shah PM, Samson P, et al. Treatment of stage I non-small cell lung cancer: what’s trending? J Thorac Cardiovasc Surg. 2017;154:1080–7.
Hu SY, Hsieh MS, Hsu HH, et al. Correlation of tumor spread through air spaces and clinicopathological characteristics in surgically resected lung adenocarcinomas. Lung Cancer. 2018;126:189–93.
Lin MW, Huang YL, Yang CY, et al. The differences in clinicopathologic and prognostic characteristics between surgically resected peripheral and central lung squamous cell carcinoma. Ann Surg Oncol. 2019;26:217–29.
Bai JH, Hsieh MS, Liao HC, et al. Prediction of pleural invasion using different imaging tools in non-small cell lung cancer. Ann Transl Med. 2019;7:33.
Lee PN, Forey BA, Coombs KJ, et al. Time trends in never smokers in the relative frequency of the different histological types of lung cancer, in particular adenocarcinoma. Regul Toxicol Pharmacol. 2016;74:12–22.
Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer—major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:138–55.
Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol. 2011;6:751–6.
Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60.
Hung WT, Hung MH, Wang ML, et al. Nonintubated thoracoscopic surgery for lung tumor: seven years’ experience with 1,025 cases. Ann Thorac Surg. 2019;107(6):1607–12.
Fiorelli A, Caronia FP, Daddi N, et al. Sublobar resection versus lobectomy for stage I non-small cell lung cancer: an appropriate choice in elderly patients? Surg Today. 2016;46:1370–82.
Gu C, Wang R, Pan X, et al. Sublobar resection versus lobectomy in patients aged ≤ 35 years with stage IA non-small cell lung cancer: a SEER database analysis. J Cancer Res Clin Oncol. 2017;143:2375–82.
Khullar OV, Liu Y, Gillespie T, et al. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer: an analysis from the national cancer data base. J Thorac Oncol. 2015;10:1625–33.
Kodama K, Higashiyama M, Okami J, et al. Oncologic outcomes of segmentectomy versus lobectomy for clinical T1a N0 M0 non-small cell lung cancer. Ann Thorac Surg. 2016;101:504–11.
Koike T, Kitahara A, Sato S, et al. Lobectomy versus segmentectomy in radiologically pure solid small-sized non-small cell lung cancer. Ann Thorac Surg. 2016;101:1354–60.
Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg. 2013;146:358–64.
Dziedzic DA, Rudzinski P, Langfort R, et al. Risk factors for local and distant recurrence after surgical treatment in patients with non-small-cell lung cancer. Clin Lung Cancer. 2016;17:157–67.
Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest. 2005;128:237–45.
Tsutani Y, Mimura T, Kai Y, et al. Outcomes after lobar versus sublobar resection for clinical stage I non-small cell lung cancer in patients with interstitial lung disease. J Thorac Cardiovasc Surg. 2017;154:1089–96.
Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg. 2010;251:550–4.
Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg. 2003;125:924–8.
Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg. 1994;107:1087–93 (discussion 93-94).
Keenan RJ, Landreneau RJ, Maley RH, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg. 2004;78:228–33 (discussion 228-33).
Kodama K, Doi O, Higashiyama M, Yokouchi H. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg. 1997;114:347–53.
Subramanian M, McMurry T, Meyers BF, et al. Long-term results for clinical stage IA lung cancer-comparing lobectomy and sublobar resection. Ann Thorac Surg. 2018;106:375–81.
Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28:928–35.
Wolf AS, Swanson SJ, Yip R, et al. The impact of margins on outcomes after wedge resection for stage I non-small cell lung cancer. Ann Thorac Surg. 2017;104:1171–8.
Passlick B, Kubuschock B, Sienel W, et al. Mediastinal lymphadenectomy in non-small cell lung cancer: effectiveness in patients with or without nodal micrometastases—results of a preliminary study. Eur J Cardiothorac Surg. 2002;21:520–6.
Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg. 2000;70:358–65 (discussion 65-66).
Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141:662–70.
Flores RM, Nicastri D, Bauer T, et al. Computed tomography screening for lung cancer: mediastinal lymph node resection in stage IA nonsmall cell lung cancer manifesting as subsolid and solid nodules. Ann Surg. 2017;265:1025–33.
Yendamuri S, Dhillon SS, Groman A, et al. Effect of the number of lymph nodes examined on the survival of patients with stage I non-small cell lung cancer who undergo sublobar resection. J Thorac Cardiovasc Surg. 2018;156:394–402.
Huang X, Wang J, Chen Q, et al. Mediastinal lymph node dissection versus mediastinal lymph node sampling for early stage non-small cell lung cancer: a systematic review and meta-analysis. PLoS ONE. 2014;9:e109979.
Saji H, Matsubayashi J, Akata S, et al. Correlation between whole tumor size and solid component size on high-resolution computed tomography in the prediction of the degree of pathologic malignancy and the prognostic outcome in primary lung adenocarcinoma. Acta Radiol. 2015;56:1187–95.
Kamiya S, Iwano S, Umakoshi H, et al. Computer-aided volumetry of part-solid lung cancers by using CT: solid component size predicts prognosis. Radiology. 2018;287:1030–40.
Maeda R, Yoshida J, Ishii G, et al. Risk factors for tumor recurrence in patients with early-stage (stage I and II) non-small cell lung cancer: patient selection criteria for adjuvant chemotherapy according to the seventh edition TNM classification. Chest. 2011;140:1494–502.
Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–4.
Wang S, Zhang B, Qian J, et al. Proposal on incorporating lymphovascular invasion as a T-descriptor for stage I lung cancer. Lung Cancer. 2018;125:245–52.
Nakagawa K, Watanabe SI, Kunitoh H, et al. The Lung Cancer Surgical Study Group of the Japan Clinical Oncology Group: past activities, current status and future direction. Jpn J Clin Oncol. 2017;47:194–9.
Kent MS, Mandrekar SJ, Landreneau R, et al. A Nomogram to predict recurrence and survival of high-risk patients undergoing sublobar resection for lung cancer: an analysis of a multicenter prospective study (ACOSOG Z4032). Ann Thorac Surg. 2016;102:239–46.
Acknowledgment
This study was supported by research grants from the National Taiwan University Hospital, Taipei, Taiwan (NTUH MS-419, NTUH 108-N4298) and the Ministry of Science and Technology, Taiwan (MOST 107-2221-E-002-080-MY3, 107-2314-B-002-247-MY3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chiang, XH., Hsu, HH., Hsieh, MS. et al. Propensity-Matched Analysis Comparing Survival After Sublobar Resection and Lobectomy for cT1N0 Lung Adenocarcinoma. Ann Surg Oncol 27, 703–715 (2020). https://doi.org/10.1245/s10434-019-07974-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07974-9