Abstract
Background
Traditional neoadjuvant therapy for esophageal cancer has used chemoradiation doses greater than 45 Gy. This study aimed to examine the dose of preoperative radiation in relation to the pathologic complete response (pCR) rate and overall survival (OS) for patients with resectable esophageal cancer.
Methods
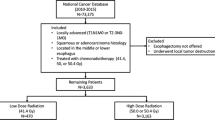
The National Cancer Database was queried for all patients with esophageal or gastroesophageal junction cancer who received neoadjuvant chemoradiation (CRT) followed by esophagectomy between 2006 and 2015. The radiation doses were divided into four ranges based on Grays (Gy) received: less than 39.6 Gy, 39.60–44.99 Gy, 45–49.99 Gy, and 50 Gy or more.
Results
The inclusion criteria were met by 10,293 patients. All patients received neoadjuvant CRT, with 689 patients (6.7%) receiving less than 39.6 Gy, 973 patients (9.5%) receiving 39.6–44.9 Gy, 3837 patients (37.3%) receiving 45–49.9 Gy, and 4794 patients (46.6%) receiving 50 Gy or more. The overall pCR rate was 17.2% (1769/10,293) and was significantly lower for those who received less than 39.6 Gy of radiation than for those who received 39.6 Gy or more (13.9% [96/689] vs. 17.4% [1673/9604]; p = 0.017). The median OS of 37.2 months was significantly better for those who received 39.6 Gy or more than for those who received less than 39.6 Gy (38 vs. 29.6 months (p < 0.0001). The pCR and OS did not differ between the three higher radiation doses (39.6–44.9 vs. 45–49.9 Gy vs. ≥ 50 Gy; pCR [p = 0.1] vs. OS [p = 0.097]). The patients who received 39.6–44.9 Gy were propensity matched with those who received 45 Gy or more of radiation. There remained no difference in pCR (p = 0.375) or OS (p = 0.957).
Conclusions
In the United States, the heterogeneity in neoadjuvant CRT dosing is significant, with 84% of patients receiving more than 45 Gy. The benefit of neoadjuvant CRT in terms of pCR and overall survival is seen with doses of 39.6 Gy or more, but not with doses higher than 45 Gy.



Similar content being viewed by others
References
Van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Cao XF, He XT, Ji L, Xiao J, Lv J. Effects of neoadjuvant radiochemotherapy on pathological staging and prognosis for locally advanced esophageal squamous cell carcinoma. Dis Esophagus. 2009;22:477–81.
Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–92.
Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–7.
Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–13.
Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the esophagus: a randomized controlled phase III trial. Lancet Oncol. 2005;6:659–68.
Tai P, Yu E. Esophageal cancer management controversies: radiation oncology point of view. World J Gastrointest Oncol. 2014;6:263–74.
Minsky BD, Pajak TF, Pisansky TM, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74.
National Cancer Database. American College of Surgeons. Retrieved 11 Jan 2019 at https://www.facs.org/quality-programs/cancer/ncdb.
Radiation Therapy Oncology Group 1010. A phase III trial evaluating the addition of trastuzumab to trimodality treatment of HER2: overexpressing esophageal adenocarcinoma. In: Radiation Therapy Oncology Group. https://clinicaltrials.gov/ct2/show/NCT01196390?term=RTOG+1010&rank=1. Accessed 11 Jan 2019.
Cancer and Leukemia Group B; National Cancer Institute. PET Scan Imaging in Assessing Response in Patients with Esophageal Cancer Receiving Combination Chemotherapy. In: ClinicalTrials.gov. National Library of Medicine (US), Bethesda, MD. http://clinicaltrials.gov/show/NCT01333033. Accessed 11 Jan 2019.
Ordu AD, Nieder C, Geninitz H, et al. Association between radiation dose and pathologic complete response after preoperative radiochemotherapy in esophageal squamous cell cancer. Anticancer Res. 2014;34:7255–61.
Singla S, Gabriel E, Alnaji R, et al. Complete pathologic response is independent of the timing of esophagectomy following neoadjuvant chemoradiation for esophageal cancer. J Gastrointest Oncol. 2018;9:73–9.
Murphy MB, Xiao L, Patel VR, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival. The University of Texas MD Anderson Cancer Center experience. Cancer. 2017;123:4106–13.
Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemo-radiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–9.
Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–7.
Suh YG, Lee IJ, Koom WS, et al. High-dose versus standard-dose radiotherapy with concurrent chemotherapy in stages II–III esophageal cancer. Jpn J Clin Oncol. 2014;44:534–40.
He L, Allen PK, Potter A, et al. Reevaluating the optimal radiation dose for definitive chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Oncol. 2014;9:1398–405.
He L, Allen PK, Potter A, et al. Reevaluating radiation dose for definitive chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Oncol. 2014;9:1398–405.
Venkat PS, Shridhar R, Naghavi AO, et al. Dose-escalated neoadjuvant chemoradiotherapy with dose-painting intensity-modulated radiation therapy and improved pathologic complete response in locally advanced esophageal cancer. Dis Esophagus. 2017;30:1–9.
Lin S H, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:1078–85.
Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32:2416–22.
Reynolds JV, Ravi N, Hollywood D, et al. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. J Thorac Cardiovasc Surg. 2006;132:549–55.
FREGAT Working Group. Impact of neoadjuvant chemoradiotherapy on postoperative outcomes after esophageal cancer resection: results of a European multicenter study. Ann Surg. 2014;260:764–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Christopher W. Towe, MD, reports that he is a consultant for Zimmer Biomet, SigMedical, Atricure, and Medtronic, but that these relationships did affect this study or the accuracy of the data analysis. The other authors have no disclosures, sources of funding, or financial relationships to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Worrell, S.G., Towe, C.W., A. Dorth, J. et al. Higher Doses of Neoadjuvant Radiation for Esophageal Cancer Do Not Affect the Pathologic Complete Response Rate or Survival: A Propensity-Matched Analysis. Ann Surg Oncol 27, 500–508 (2020). https://doi.org/10.1245/s10434-019-07849-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07849-z