Abstract
Background
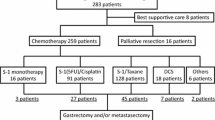
A retrospective study was performed to evaluate the predictive factors for performing curative-intent surgery and prognostic factors for long-term survival of patients undergoing surgery for stage IV gastric cancer.
Patients and Methods
Between 2001 and 2017, 271 patients with stage IV gastric cancer with distant metastasis who underwent systemic chemotherapy were enrolled. Logistic regression analysis was performed to evaluate predictive factors for curative-intent surgery. Cox proportional hazards regression model was applied for patients who were subsequently treated with curative-intent surgery to identify prognostic factors for long-term survival.
Results
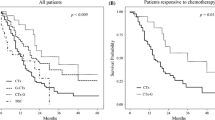
Curative-intent surgery was performed in 48 patients (17.7%). Median survival time was significantly longer in the surgery group than in the nonsurgery group (53 vs. 11 months, p < 0.0001). R0 resection was performed in 35 patients (72.9%). The three-year overall survival (OS) rates of the R0, R1, and R2 surgery groups were 75.4%, 33.3%, and 25.0%, respectively (p = 0.0002). Logistic regression analysis revealed that lymphogenous distant metastasis alone (odds ratio = 3.276, p = 0.004), positive lavage cytology alone (6.394, 0.014), doublet or triplet chemotherapy (4.064, 0.034), and high Glasgow prognostic score (0.276, 0.001) were independent predictive factors for performing curative-intent surgery. Among patients undergoing surgery, the Cox proportional hazards regression model for OS showed that R0 surgery was an independent prognostic factor for favorable OS (hazard ratio 0.188, p = 0.022).
Conclusions
Patients with lymphogenous distant metastasis alone, P0CY1 alone, good immunonutritional status, and doublet/triplet chemotherapy are candidates for performing effective curative-intent surgery. R0 surgery is crucial for improving long-term survival after surgery.



Similar content being viewed by others
References
Ferlay JEM, Lam F, Colombet M, Mery L, Pineros M, Znaor A, Soerjomataram I, Bray F. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer 2018. https://gco.iarc.fr/today.
Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376(9742):687–97.
Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008; 9(3):215–21.
Sawaki A, Ohashi Y, Omuro Y, et al. Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer. 2012; 15(3):313–22.
Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol. 2015; 26(1):141–8.
Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016; 17(3):309–18.
Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011; 14(2):97–100.
Lordick F, Siewert JR. Recent advances in multimodal treatment for gastric cancer: a review. Gastric Cancer. 2005; 8(2):78–85.
Mahar AL, Coburn NG, Karanicolas PJ, Viola R, Helyer LK. Effective palliation and quality of life outcomes in studies of surgery for advanced, non-curative gastric cancer: a systematic review. Gastric Cancer. 2012; 15(Suppl 1):S138–45.
Shen L, Shan YS, Hu HM, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013; 14(12):e535–47.
Kodera Y. Surgery with curative intent for stage IV gastric cancer: is it a reality of illusion? Ann Gastroenterol Surg. 2018; 2(5):339–47.
Chan DY, Syn NL, Yap R, et al. Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. J Gastrointest Surg. 2017; 21(3):425–33.
Einama T, Abe H, Shichi S, et al. Long-term survival and prognosis associated with conversion surgery in patients with metastatic gastric cancer. Mol Clin Oncol. 2017; 6(2):163–6.
Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017; 20(Suppl 1):128–34.
Ishigami S, Natsugoe S, Nakajo A, et al. Salvage gastrectomy following a combination of biweekly paclitaxel and S-1 for stage IV gastric cancer. J Gastrointest Surg. 2008; 12(8):1370–75.
Mieno H, Yamashita K, Hosoda K, et al. Conversion surgery after combination chemotherapy of docetaxel, cisplatin and S-1 (DCS) for far-advanced gastric cancer. Surg Today. 2017; 47(10):1249–58.
Sato Y, Ohnuma H, Nobuoka T, et al. Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S-1 (DCS) chemotherapy: a multi-institutional retrospective study. Gastric Cancer. 2017; 20(3):517–26.
Satoh S, Okabe H, Teramukai S, et al. Phase II trial of combined treatment consisting of preoperative S-1 plus cisplatin followed by gastrectomy and postoperative S-1 for stage IV gastric cancer. Gastric Cancer. 2012; 15(1):61–9.
Yamaguchi K, Yoshida K, Tanahashi T, et al. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018; 21(2):315–23.
Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016; 19(2):329–38.
Japanese Gastric Cancer A. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14(2):101–12.
Japanese Gastric Cancer A. Japanese classification of gastric carcinoma: 2nd English Edition. Gastric Cancer. 1998; 1(1):10–24.
Sakata Y, Ohtsu A, Horikoshi N, et al. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998; 34(11):1715–20.
Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014; 140(2):319–28.
Kunisaki C, Takahashi M, Makino H, et al. Phase II study of biweekly docetaxel and S-1 combination chemotherapy as first-line treatment for advanced gastric cancer. Cancer Chemother Pharmacol. 2011; 67(6):1363–68.
Kunisaki C, Takahashi M, Nagahori Y, et al. Phase I study of biweekly docetaxel and S-1 combination chemotherapy for advanced gastric cancer. Anticancer Res. 2008; 28(4c):2473–78.
Takahari D, Mizusawa J, Koizumi W, Hyodo I, Boku N. Validation of the JCOG prognostic index in advanced gastric cancer using individual patient data from the SPIRITS and G-SOX trials. Gastric Cancer. 2017; 20(5):757–63.
Sato K, Kunisaki C, Kosaka T, et al. Multicenter phase II study of capecitabine plus cisplatin as first-line therapy for human epidermal growth factor receptor 2-negative advanced gastric cancer: Yokohama Clinical Oncology Group Study YCOG1107. Cancer Chemother Pharmacol. 2017; 80(5):939–43.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240(2):205–13.
Association JGC. Japanese gastric cancer treatment guidelines 2018 (ver. 5)2018.
Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984; 85(9):1001–5.
Terashima M. Conversion therapy for gastric cancer: who can make conversion as successful as Goromaru? Gastric Cancer. 2016; 19(3):685–6.
Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014; 101(6):653–60.
Kunisaki C, Shimada H, Yamaoka H, et al. Indications for paraaortic lymph node dissection in gastric cancer patients with paraaortic lymph node involvement. Hepatogastroenterology. 2000; 47(32):586–9.
Kodera Y, Ito S, Mochizuki Y, et al. Long-term follow up of patients who were positive for peritoneal lavage cytology: final report from the CCOG0301 study. Gastric Cancer. 2012; 15(3):335–7.
Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004; 90(9):1704–6.
Kimura J, Kunisaki C, Makino H, et al. Evaluation of the Glasgow Prognostic Score in patients receiving chemoradiotherapy for stage III and IV esophageal cancer. Dis Esophagus. 2016; 29(8):1071–80.
Kunisaki C, Takahashi M, Ono HA, et al. Inflammation-based prognostic score predicts survival in patients with advanced gastric cancer receiving biweekly docetaxel and s-1 combination chemotherapy. Oncology. 2012; 83(4):183–91.
Miki C, Konishi N, Ojima E, et al. C-reactive protein as a prognostic variable that reflects uncontrolled up-regulation of the IL-1-IL-6 network system in colorectal carcinoma. Dig Dis Sci. 2004; 49(6):970–6.
Jee SH, Shen SC, Chiu HC, Tsai WL, Kuo ML. Overexpression of interleukin-6 in human basal cell carcinoma cell lines increases anti-apoptotic activity and tumorigenic potency. Oncogene. 2001; 20(2):198–208.
Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998; 34(4):503–9.
Sato S, Kunisaki C, Suematsu H, et al. Impact of sarcopenia in patients with unresectable locally advanced esophageal cancer receiving chemoradiotherapy. In Vivo. 2018; 32(3):603–10.
Fukuchi M, Ishiguro T, Ogata K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015; 22(11):3618–24.
Yamamoto M, Kawano H, Yamaguchi S, et al. Comparison of neoadjuvant chemotherapy to surgery followed by adjuvant chemotherapy in japanese patients with peritoneal lavage cytology positive for gastric carcinoma. Anticancer Res. 2015; 35(9):4859–63.
Funding
This work is not supported by any financial assistance grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sato, S., Kunisaki, C., Tanaka, Y. et al. Curative-Intent Surgery for Stage IV Advanced Gastric Cancer: Who Can Undergo Surgery and What Are the Prognostic Factors for Long-Term Survival?. Ann Surg Oncol 26, 4452–4463 (2019). https://doi.org/10.1245/s10434-019-07790-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07790-1