Abstract
Background
The small bowel and pancreas are the most common primary sites of neuroendocrine tumors (NETs) giving rise to metastatic disease. Some patients with small bowel NETs (SBNETs) present with synchronous or metachronous pancreatic NETs (PNETs), and it is unclear whether these are separate primaries or metastases from one site to the other.
Methods
A surgical NET database including patients undergoing operations for SBNETs or PNETs was reviewed. Patients with synchronous or metachronous tumors in both the small bowel and pancreas were identified, and available tissues from primary tumors and metastases were examined using a 4-gene quantitative polymerase chain reaction (qPCR) and immunohistochemistry (IHC) panel developed for evaluating NETs of unknown primary.
Results
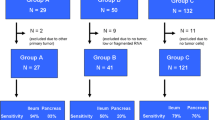
Of 338 patients undergoing exploration, 11 had NETs in both the small bowel and pancreas. Tissues from 11 small bowel tumors, 9 pancreatic tumors, and 10 metastases were analyzed. qPCR and IHC data revealed that three patients had separate SBNET and PNET primaries, and five patients had SBNETs that metastasized to the pancreas. Pancreatic tissue was unavailable in two patients, and qPCR and IHC gave discrepant results in one patient.
Conclusions
NETs in both the small bowel and pancreas were found in 3% of our patients. In nearly two-thirds of evaluable patients, the pancreatic tumor was a metastasis from the SBNET primary, while in the remaining one-third of patients it represented a separate primary. Determining the origin of these tumors can help guide the choice of systemic therapy and surgical management.



Similar content being viewed by others
References
Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–42.
Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121(4):589–97.
Riihimaki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer. 2016;139(12):2679–86.
Kunikowska J, Pawlak D, Kolasa A, Mikolajczak R, Krolicki L. A frequency and semiquantitative analysis of pathological 68Ga DOTATATE PET/CT uptake by primary site-dependent neuroendocrine tumor metastasis. Clin Nucl Med. 2014;39(10):855–61.
Agarwal R, Szalkiewicz ER, Warner RR, et al. Multiple endocrine neoplasia type 1 associated with a new mutation in the menin gene and a midgut neuroendocrine tumor. Pancreas. 2014;43(1):145–6.
Kotteas EA, Syrigos KN, Saif MW. Profile of capecitabine/temozolomide combination in the treatment of well-differentiated neuroendocrine tumors. Onco Targets Ther. 2016;9:699–704.
Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–13.
Givi B, Pommier SJ, Thompson AK, Diggs BS, Pommier RF. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery. 2006;140(6):891–97 (discussion 897–898).
Lewis A, Raoof M, Ituarte PHG, et al. Resection of the primary gastrointestinal neuroendocrine tumor improves survival with or without liver treatment. Ann Surg. https://doi.org/10.1097/sla.0000000000002809.
Almond LM, Hodson J, Ford SJ, et al. Role of palliative resection of the primary tumour in advanced pancreatic and small intestinal neuroendocrine tumours: a systematic review and meta-analysis. Eur J Surg Oncol. 2017;43(10):1808–15.
Bellizzi AM. Assigning site of origin in metastatic neuroendocrine neoplasms: a clinically significant application of diagnostic immunohistochemistry. Adv Anat Pathol. 2013;20(5):285–314.
Maxwell JE, Sherman SK, Stashek KM, O’Dorisio TM, Bellizzi AM, Howe JR. A practical method to determine the site of unknown primary in metastatic neuroendocrine tumors. Surgery. 2014;156(6):1359–65 (discussion 1365–1356).
Sherman SK, Maxwell JE, Carr JC, et al. Gene expression accurately distinguishes liver metastases of small bowel and pancreas neuroendocrine tumors. Clin Exp Metastasis. 2014;31(8):935–44.
Carr JC, Sherman SK, Wang D, et al. Overexpression of membrane proteins in primary and metastatic gastrointestinal neuroendocrine tumors. Ann Surg Oncol. 2013;20(Suppl 3):S739–46.
Czeczok TW, Stashek KM, Maxwell JE, et al. Clusterin in neuroendocrine epithelial neoplasms: absence of expression in a well-differentiated tumor suggests a jejunoileal origin. Appl Immunohistochem Mol Morphol. 2018;26(2):94–100.
Tsunenari T, Aosasa S, Ogata S, et al. Synchronous neuroendocrine tumors in both the pancreas and ileum: a case report. Int J Surg Case Rep. 2016;22:47–50.
Masuoka Y, Furukawa D, Yazawa N, et al. Rectal neuroendocrine tumor with synchronous pancreatic metastasis: a case report. Tokai J Exp Clin Med. 2018;43(2):38–44.
Milanetto AC, Zurcher A-LG, Da Broi M, Pedrazzoli S, Pasquali C. Metastases to the pancreas from small intestinal neuroendocrine tumors [abstract no. P3-200]. Pancreatology. 2018;18(4):S143.
Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132(6):931–9.
Nakamura E, Shimizu M, Itoh T, Manabe T. Secondary tumors of the pancreas: clinicopathological study of 103 autopsy cases of Japanese patients. Pathol Int. 2001;51(9):686–90.
Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004;444(6):527–35.
Lamarca A, Elliott E, Barriuso J, et al. Chemotherapy for advanced non-pancreatic well-differentiated neuroendocrine tumours of the gastrointestinal tract, a systematic review and meta-analysis: a lost cause? Cancer Treat Rev. 2016;44:26–41.
Angelousi A, Kaltsas G, Koumarianou A, Weickert MO, Grossman A. Chemotherapy in NETs: when and how. Rev Endocr Metab Disord. 2017;18(4):485–97.
Woltering EA, Voros BA, Beyer DT, et al. Aggressive surgical approach to the management of neuroendocrine tumors: a report of 1,000 surgical cytoreductions by a single institution. J Am Coll Surg. 2017;224(4):434–47.
Sherman SK, Maxwell JE, O’Dorisio MS, O’Dorisio TM, Howe JR. Pancreastatin predicts survival in neuroendocrine tumors. Ann Surg Oncol. 2014;21(9):2971–80.
Khan MS, Kirkwood A, Tsigani T, et al. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J Clin Oncol. 2013;31(3):365–72.
Rosales-Velderrain A, Bowers SP, Goldberg RF, et al. National trends in resection of the distal pancreas. World J Gastroenterol. 2012;18(32):4342–9.
Smith JK, Ng SC, Hill JS, et al. Complications after pancreatectomy for neuroendocrine tumors: a national study. J Surg Res. 2010;163(1):63–8.
He J, Ahuja N, Makary MA, et al. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB (Oxford). 2014;16(1):83–90.
Acknowledgment
This work was supported by National Institutes of Health T32 Grant CA148062-0 (AS), National Cancer Institute SPORE Grant P50 CA174521-01 (JH, TO, AB, JD), and National Institutes of Health Grant CTSA UL1TR002537 (RedCap database). In addition, this work was presented as a poster at the Society of Surgical Oncology 2018 Annual Cancer Symposium, Chicago, IL, USA, 22 March 2018.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Scott, A.T., Pelletier, D., Maxwell, J.E. et al. The Pancreas as a Site of Metastasis or Second Primary in Patients with Small Bowel Neuroendocrine Tumors. Ann Surg Oncol 26, 2525–2532 (2019). https://doi.org/10.1245/s10434-019-07370-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07370-3