Abstract
Background
CD44 isoforms serve as a marker for cancer stem cells. CD44 variant 9 (CD44v9) contributes to the defense against reactive oxygen species, resulting in resistance to chemoradiotherapy. However, the significance of CD44v9 in patients with lung adenocarcinoma is unknown.
Methods
We used immunohistochemical analysis to retrospectively analyze CD44v9 expression in 268 surgically resected lung adenocarcinomas and investigated the association between CD44v9 expression and patients’ clinicopathological features.
Results
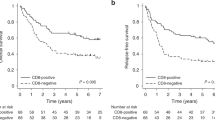
The expression of CD44v9 in 193 of 268 (72.0%) patients was significantly associated with early-stage cancer, low-grade tumors, absence of vessel and pleural invasion, and a mutated epidermal growth factor receptor (EGFR) gene. Multivariate logistic analysis revealed that CD44v9 expression was significantly associated with early-stage disease [odds ratio (OR) 0.29, 95% confidence interval (CI) 0.14–0.59; p < 0.001] and mutant EGFR (OR 2.53, 95% CI 1.06–6.04; p = 0.036). The percentage of CD44v9-positive tumors was higher in the earlier stages of disease; however, there was no significant difference in the survival of patients in each stage of disease who had positive or negative CD44v9 expression.
Conclusion
CD44v9 was highly expressed in EGFR-mutant tumors, particularly in early-stage lung adenocarcinoma, suggesting that CD44v9 expression may play an important role in EGFR-mutant tumors.



Similar content being viewed by others
References
Oxnard GR, Binder A, Janne PA. New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1097–04.
Takada K, Toyokawa G, Shoji F, Okamoto T, Maehara Y. The significance of the PD-L1 expression in non-small-cell lung cancer: trenchant double swords as predictive and prognostic markers. Clin Lung Cancer. 2018;19(2):120–29.
Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Codd AS, Kanaseki T, Torigo T, Tabi Z. Cancer stem cells as targets for immunotherapy. Immunology. 2018;153(3):304–14.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11.
Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4(1):33–45.
Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nature reviews. Cancer. 2011;11(4):254–67.
Ishii H, Saitoh M, Sakamoto K, et al. Epithelial splicing regulatory proteins 1 (ESRP1) and 2 (ESRP2) suppress cancer cell motility via different mechanisms. J Biol Chem. 2014;289(40):27386–99.
Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387–400.
Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–83.
Sosulski A, Horn H, Zhang L, et al. CD44 splice variant v8-10 as a marker of serous ovarian cancer prognosis. PLoS ONE. 2016;11(6):e0156595.
Hirata K, Suzuki H, Imaeda H, et al. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. Br J Cancer. 2013;109(2):379–86.
Aso T, Matsuo M, Kiyohara H, et al. Induction of CD44 variant 9-expressing cancer stem cells might attenuate the efficacy of chemoradioselection and worsens the prognosis of patients with advanced head and neck cancer. PLoS ONE. 2015;10(3):e0116596.
Kobayashi K, Matsumoto H, Matsuyama H, et al. Clinical significance of CD44 variant 9 expression as a prognostic indicator in bladder cancer. Oncol Rep. 2016;36(5):2852–60.
Shitara K, Doi T, Nagano O, et al. Phase 1 study of sulfasalazine and cisplatin for patients with CD44v-positive gastric cancer refractory to cisplatin (EPOC1407). Gastric cancer. 2017;20(6):1004–09.
Otsubo K, Nosaki K, Imamura CK, et al. Phase I study of salazosulfapyridine in combination with cisplatin and pemetrexed for advanced non-small-cell lung cancer. Cancer Sci. 2017;108(9):1843–49.
Kohno M, Okamoto T, Suda K, et al. Prognostic and therapeutic implications of aromatase expression in lung adenocarcinomas with EGFR mutations. Clin Cancer Res. 2014;20(13):3613–22.
Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–68.
Yamaguchi A, Urano T, Goi T, et al. Expression of a CD44 variant containing exons 8 to 10 is a useful independent factor for the prediction of prognosis in colorectal cancer patients. J Clin Oncol. 1996;14(4):1122–27.
Sato S, Miyauchi M, Takekoshi T, et al. Reduced expression of CD44 variant 9 is related to lymph node metastasis and poor survival in squamous cell carcinoma of tongue. Oral Oncol. 2000;36(6):545–49.
Go SI, Ko GH, Lee WS, et al. CD44 variant 9 serves as a poor prognostic marker in early gastric cancer, but not in advanced gastric cancer. Cancer Res Treat. 2016;48(1):142–52.
Brown RL, Reinke LM, Damerow MS, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121(3):1064–74.
Mashita N, Yamada S, Nakayama G, et al. Epithelial to mesenchymal transition might be induced via CD44 isoform switching in colorectal cancer. J Surg Oncol. 2014;110(6):745–51.
Larsen JE, Nathan V, Osborne JK, et al. ZEB1 drives epithelial-to-mesenchymal transition in lung cancer. J Clin Invest. 2016;126(9):3219–35.
Kawano Y, Iwama E, Tsuchihashi K, et al. CD44 variant-dependent regulation of redox balance in EGFR mutation-positive non-small cell lung cancer: a target for treatment. Lung Cancer. 2017;113:72–78.
Suda K, Murakami I, Yu H, et al. CD44 facilitates epithelial to mesenchymal transition phenotypic change at acquisition of resistance to EGFR kinase inhibitors in lung cancer. Mol Cancer Ther. 2018;17(10):2257–65.
Acknowledgments
The authors thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
DISCLOSURE
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1
Expression of CD44 in lung adenocarcinoma demonstrating representative (a) negative and (b) positive cases (TIFF 480 kb)
Fig. 2
Mosaic plot of data for a proportion of CD44v9 Allred scores at each stage (TIFF 70 kb)
Fig. 3
Kaplan–Meier analysis of association of stage-specific CD44v9 expression in relation to progression-free and overall survival (TIFF 94 kb)
Rights and permissions
About this article
Cite this article
Akamine, T., Tagawa, T., Ijichi, K. et al. The Significance of CD44 Variant 9 in Resected Lung Adenocarcinoma: Correlation with Pathological Early-Stage and EGFR Mutation. Ann Surg Oncol 26, 1544–1551 (2019). https://doi.org/10.1245/s10434-018-07137-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-07137-2