Abstract
Background
Neoadjuvant chemotherapy (NAC) is used to convert patients with inoperable locally advanced breast cancer (LABC) to operability, but has not traditionally been used to avoid mastectomy or axillary dissection in this subset.
Objective
The purpose of this study was to determine the rates of pathologic complete response (pCR) in LABC patients, and identify factors predictive of pCR to determine if responding patients might be suitable for limited surgery.
Methods
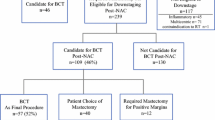
From 2006 to 2016, 1522 patients received NAC followed by surgery; 321 had advanced disease in the breast (cT4) and/or in the nodes (cN2/N3). pCR rates were assessed by T and N stage, and receptor subtype.
Results
Of 321 LABC patients, 223 were cT4, 77 were cN2, and 82 were cN3. Forty-three percent were hormone receptor (HR) positive/human epidermal growth factor receptor 2 (HER2) negative (HR+/HER2−), 23% were triple negative, and 34% were HER2+. The overall pCR rate was 25% and differed by receptor subtype (HR+/HER2− 7%, triple negative 23%, HER2+ 48%; p < 0.001). Breast pCR occurred in 27% of patients and was similar in T4 versus non-T4 disease (29% vs. 22%; p = 0.26). Nodal pCR was achieved in 38% of cN+ patients and did not differ by nodal stage (cN1 43%, cN2 36%, cN3 32%; p = 0.23). Nodal pCR was significantly more common than breast pCR (p = 0.014) across all tumor subtypes. Receptor subtype was the only predictor of overall pCR (p < 0.001).
Conclusion
In patients with LABC, pCR after NAC was seen in 25%, and did not differ by T or N stage. Tumor biology, but not extent of disease, predicted pCR. Studies assessing the feasibility of surgical downstaging with NAC in LABC patients are warranted.
Similar content being viewed by others
References
Hunt KK, Yi M, Mittendorf EA, Guerrero C, Babiera GV, Bedrosian I, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009;250(4):558–66.
Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, et al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol. 2016;23(11):3467–74.
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85.
Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260(4):608–14; discussion 14–6.
Chia S, Swain SM, Byrd DR, Mankoff DA. Locally advanced and inflammatory breast cancer. J Clin Oncol. 2008;26(5):786–90.
Hidar S, Bibi M, Gharbi O, Tebra S, Trabelsi A, Korbi S, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in inflammatory breast cancer. Int J Surg. 2009;7(3):272–75.
Mocellin S, Goldin E, Marchet A, Nitti D. Sentinel node biopsy performance after neoadjuvant chemotherapy in locally advanced breast cancer: a systematic review and meta-analysis. Int J Cancer. 2016;138(2):472–80.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Version 2. Available at: https://www.nccn.org. (2016). Accessed 6 Mar 2017.
Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21(22):4165–74.
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–84.
Shen J, Valero V, Buchholz TA, Singletary SE, Ames FC, Ross MI, et al. Effective local control and long-term survival in patients with T4 locally advanced breast cancer treated with breast conservation therapy. Ann Surg Oncol. 2004;11(9):854–60.
Murphy BL, Hoskin TL, Boughey JC, Degnim AC, Carter JM, Glazebrook KN, et al. Contemporary operative management of T4 breast cancer. Surgery. 2016;160(4):1059–69.
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102.
Lobbes MB, Prevos R, Smidt M, Tjan-Heijnen VC, van Goethem M, Schipper R, et al. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging. 2013;4(2):163–75.
Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64.
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18.
Galimberti V, Ribeiro Fontana SK, Maisonneuve P, Steccanella F, Vento AR, Intra M, et al. Sentinel node biopsy after neoadjuvant treatment in breast cancer: five-year follow-up of patients with clinically node-negative or node-positive disease before treatment. Eur J Surg Oncol. 2016;42(3):361–8.
Disclosures
The preparation of this study was funded in part by NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center, and was presented as a poster presentation at the 70th Society of Surgical Oncology Annual Cancer Symposium, 15–18 March, 2017, Seattle, WA, USA. This study was supported by Judy Guitelman, Dan Epstein, and the Daniel J. Epstein Family Foundation. Lori F. Gentile, George Plitas, Emily C. Zabor, Michelle Stempel, Monica Morrow, and Andrea V. Barrio have no conflict of interest disclosures to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gentile, L.F., Plitas, G., Zabor, E.C. et al. Tumor Biology Predicts Pathologic Complete Response to Neoadjuvant Chemotherapy in Patients Presenting with Locally Advanced Breast Cancer. Ann Surg Oncol 24, 3896–3902 (2017). https://doi.org/10.1245/s10434-017-6085-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6085-y