Abstract
Background
The outcome of gastric cancer patients with peritoneal metastasis remains poor. We treated these patients with intraperitoneal and intravenous paclitaxel plus oral S-1 (tegafur/gimeracil/oteracil), followed by gastrectomy in responders. We evaluated the clinical significance of peritoneal lavage carcinoembryonic antigen (CEA) messenger RNA (mRNA) levels as a biomarker for indication of conversion gastrectomy.
Methods
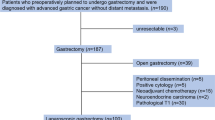
The peritoneal lavage of 68 patients who received the above regimen as induction chemotherapy was repeatedly collected via intraperitoneal access ports. Gastrectomy was considered when improvement of peritoneal metastasis was confirmed by a second laparoscopic examination with negative peritoneal cytology. CEA and porphobilinogen deaminase mRNAs were chronologically quantified using the transcription reverse-transcription concerted reaction method. The CEA mRNA index (CmRI) was calculated as CEA mRNA/porphobilinogen deaminase mRNA × 10,000.
Results
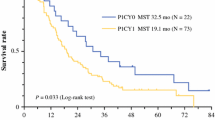
Thirty-nine patients underwent gastrectomy and 29 patients did not (median survival time, 27.8 vs. 10.7 months, respectively; P < 0.001). In gastrectomy-positive patients, the outcome largely differed according to CmRI values immediately prior to surgery. Patients with a preoperative CmRI value <100 (n = 20) were associated with a significantly longer survival than those with a preoperative CmRI value >100 (n = 19) (41.8 vs. 20.1 months, respectively; P < 0.001). A preoperative CmRI value <100 was confirmed as an independent predictor of survival for gastrectomy-positive patients in the multivariate analysis.
Conclusions
The CmRI reflects the response of peritoneal metastases to induction intraperitoneal chemotherapy. It may be a useful biomarker for indicating gastrectomy in gastric cancer patients with peritoneal metastasis.


Similar content being viewed by others
References
Yamao T, Shimada Y, Shirao K, et al. Phase II study of sequential methotrexate and 5-fluorouracil chemotherapy against peritoneally disseminated gastric cancer with malignant ascites: a report from the Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group, JCOG 9603 Trial. Jpn J Clin Oncol. 2004;34:316–22.
Imazawa M, Kojima T, Boku N, et al. Efficacy of sequential methotrexate and 5-fluorouracil (MTX/5FU) in improving oral intake in patients with advanced gastric cancer with severe peritoneal dissemination. Gastric Cancer. 2009;12:153–7.
Oh SY, Kwon HC, Lee S, et al. A Phase II study of oxaliplatin with low-dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFOX-4) for gastric cancer patients with malignant ascites. Jpn J Clin Oncol. 2007;37:930–5.
Kitayama J, Ishigami H, Yamaguchi H, et al. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol. 2014;21:539–46.
Yamaguchi H, Kitayama J, Ishigami H, et al. Breakthrough therapy for peritoneal carcinomatosis of gastric cancer: intraperitoneal chemotherapy with taxanes. World J Gastrointest Oncol. 2015;7:285–91.
Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70.
Fushida S, Kinoshita J, Kaji M, et al. Phase I/II study of intraperitoneal docetaxel plus S-1 for the gastric cancer patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2013;71:1265–72.
Fujiwara Y, Takiguchi S, Nakajima K, et al. Intraperitoneal docetaxel combined with S-1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol. 2012;105:38–42.
Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119:3354–8.
Ishigami H, Kitayama J, Otani K, et al. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer. Oncology. 2009;76:311–4.
Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017;20:128–34.
Ishii T, Fujiwara Y, Ohnaka S, et al. Rapid genetic diagnosis with the transcription-reverse transcription concerted reaction system for cancer micrometastasis. Ann Surg Oncol. 2004;11:778–85.
Murono K, Kazama S, Yamaguchi H, et al. Detection of carcinoembryonic antigen mRNA in peritoneal lavage by the transcription-reverse transcription concerted method indicates poor prognosis in patients with stage II and III colon cancer. Surgery. 2015;157:322–30.
Kodera Y, Nakanishi H, Ito S, et al. Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer. 2002;5:69–76.
Wang JY, Lin SR, Lu CY, et al. Gastric cancer cell detection in peritoneal lavage: RT-PCR for carcinoembryonic antigen transcripts versus the combined cytology with peritoneal carcinoembryonic antigen levels. Cancer Lett. 2005;223:129–35.
Fujiwara Y, Doki Y, Taniguchi H, et al. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer. 2007;10:197–204.
Emoto S, Ishigami H, Yamashita H, Yamaguchi H, Kaisaki S, Kitayama J. Clinical significance of CA125 and CA72-4 in gastric cancer with peritoneal dissemination. Gastric Cancer. 2012;15:154–61.
Takata A, Kurokawa Y, Fujiwara Y, et al. Prognostic value of CEA and CK20 mRNA in the peritoneal lavage fluid of patients undergoing curative surgery for gastric cancer. World J Surg. 2014;38:1107–11.
Li Z, Zhang D, Zhang H, Miao Z, Tang Y, Sun G, Dai D. Prediction of peritoneal recurrence by the mRNA level of CEA and MMP-7 in peritoneal lavage of gastric cancer patients. Tumour Biol. 2014;35:3463–70.
Horikawa M, Iinuma H, Inoue T, Ogawa E, Fukushima R. Clinical significance of intraperitoneal CD44 mRNA levels of magnetically separated CD45-negative EpCAM-positive cells for peritoneal recurrence and prognosis in stage II and III gastric cancer patients. Oncol Rep. 2011;25:1413–20.
Okada K, Fujiwara Y, Nakamura Y, et al. Oncofetal protein, IMP-3, a potential marker for prediction of postoperative peritoneal dissemination in gastric adenocarcinoma. J Surg Oncol. 2012;105:780–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have nothing to disclose.
Funding
This research was partially funded by a Grant from the Japan Agency for Medical Research and Development, and a KAKENHI Grant-in-Aid for Scientific Research (No. 15K10086) from the Japan Society for the Promotion of Science.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 6079 kb)
Alterations in carcinoembryonic antigen (CEA) messenger RNA (mRNA) index values during induction chemotherapy in conversion gastrectomy patients with a preoperative CEA mRNA index value of (a) <100 (n = 20) or (b) >100 (n = 19). The x-axis represents the number of days since commencing induction chemotherapy. c Alterations in CEA mRNA index values during first-line chemotherapy up until the point of switching to second-line treatment in patients who did not undergo conversion gastrectomy (n = 29)
Rights and permissions
About this article
Cite this article
Yamaguchi, H., Satoh, Y., Ishigami, H. et al. Peritoneal Lavage CEA mRNA Levels Predict Conversion Gastrectomy Outcomes after Induction Chemotherapy with Intraperitoneal Paclitaxel in Gastric Cancer Patients with Peritoneal Metastasis. Ann Surg Oncol 24, 3345–3352 (2017). https://doi.org/10.1245/s10434-017-5997-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5997-x