Abstract
Purpose
To determine whether the benefits of sentinel-node-based management (SNBM) over routine axillary clearance (RAC) persisted to 5 years.
Methods
A total of 1088 women with breast cancer less than 3 cm in diameter and clinically negative axillary nodes were randomized to SNBM with axillary clearance if the sentinel node was positive or RAC preceded by sentinel-node biopsy. The outcomes were: (1) objectively measured change in the volume of the operated and contralateral nonoperated arms; (2) the proportion with an increase in arm volume <15%; and (3) subjectively assessed arm morbidity for the domains swelling, symptoms, dysfunction, and disability. Assessments were performed at 1 and 6 months after surgery and then annually.
Results
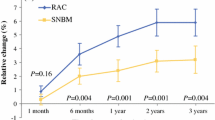
Limb volume increased progressively in the operated and nonoperated arms for 2 years and persisted unchanged to year 5, accompanied by weight gain. Correction by change in the nonoperated arm showed a mean volume increase of 70 mL in the RAC group and 26 mL in the SNBM group (P < 0.001) at 5 years. Only 28 patients (3.3%) had a corrected increase >15% from baseline (RAC 5.0% vs. SNBM 1.7%). Significant predictors were surgery type (RAC vs. SNBM), obesity, diabetes, palpable tumor, and weight gain exceeding 10% of baseline value.
Conclusions
Subjective assessments revealed persisting patient concerns about swelling and symptoms but not overall disability at 5 years. Subjective scores were only moderately correlated with volume increase. SNAC1 has demonstrated that objective morbidity and subjective morbidity persist for 5 years after surgery and that SNBM significantly lowers the risk of both.

Similar content being viewed by others
References
Gill PG, Wetzig N, Gebski V, et al, and the SNAC Trial Group. Sentinel-lymph-node-based management or routine axillary clearance? One-year outcomes of sentinel node biopsy versus axillary clearance (SNAC): a randomized controlled surgical trial. Ann Surg Oncol. 2009;16:266–75.
Elmadahm AA, Gill PG, Bochner M, Gebski V, Zannino D, Wetzig N, Campbell I, Stockler M, Ung O, Simes J, Uren R.. Identification of the Sentinel Lymph Node in the SNAC-1 Trial. ANZ J Surg. 2015;85(1–2):58–63.
Tewari N, Gill P G, Bochner M, Kollias J. Comparison of volume displacement versus circumferential upper limb measurements for lymphoedema: implications for the SNAC trial. ANZ J Surg. 2008;78:889–93.
Smith MJ, Gill PG, Wetzig N, et al. Comparing patients’ and clinicians’ assessment of outcomes in a randomised trial of sentinel node biopsy for breast cancer (the RACS SNAC trial). Breast Cancer Res Treat. 2008;117:99–109.
Wetzig N, Gill PG, Zanno D, Stockler MR, Gebski V, Ung O, Campbell I, Simes J. Sentinel-lymph-node-based management or routine axillary clearance? Three-year outcomes of the RACS sentinel node biopsy versus axillary clearance (SNAC) 1 trial. Ann Surg Oncol. 2014;16:266–75.
Mansel RE, Fallowfield LJ, Kissin M. Randomised multicentre trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98(9):599–609.
Wilke LG, McCall LM, Posther KE, Whitworth PW, Reintgen DS, Leitch AM, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13(4):491–500.
Hack TF, Kwan WB, Thomas-MacLean JL, Towers A, Miedema B, Tilley A, et al. Predictors of arm morbidity following breast cancer surgery. Psycho-Oncology. 2010;19(11):1205–12.
Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. J Natl Cancer Inst. 2001;93(2):96–111.
Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102(2):111–8.
Coen JJ, Taghian AG, Kachnic LA, Coen JJ, Assaad SI. Risk of lymphoedema after regional nodal irradiation with breast conservation therapy. Int J Radiat Oncol Biol Phys. 2003;55(5):1209–15.
Dayes IS, Levine MN, Julian JA, Pritchard KI, D’Souza DP, Kligman L, et al. Lymphoedema in women with breast cancer: characteristics of patients screened for a randomized trial. Breast Cancer Res Treat. 2008;110(2):337–42.
McLaughlin SA, Wright MJ, Morris KT. Prevalence of lymphoedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: patient perceptions and precautionary behaviours. J Clin Oncol. 2008;26(32):5220–6.
Kissin M, Querci-Della-Rovere G, Easton D, Westbury G. Risk of lymphoedema following the treatment for breast cancer. BJS. 1986;73:580–4.
Larson D, Weinstein M, Goldburg L, Silver B, Recht A, Cady Y. Oedema of the arm as a function of the extent of axillary surgery in patients with stage 1-2 carcinoma of the breast treated primary radiotherapy. Int J Radiat Oncol Biol Phys. 1986:12;1575–82.
Liljegren G, Holmburg L. Arm morbidity after sector resection and axillary dissection with or without postoperative radiotherapy in breast cancer stage 1: results from a randomised trial. Eur J Cancer. 1997;33;193–9.
Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphoedema in breast cancer patients. Breast J. 2010;16(1):48–54.
Herd-Smith A, Russo A, Muraca MG, Del Turco MR, Cardona G. Prognostic factors for lymphoedema after primary treatment of breast carcinoma. Cancer. 2001;92(7):1783–7.
Soran A, D’Angelo G, Begovic M, Ardic F, Harlak A, Samuel Wieand H, et al. Breast cancer-related lymphoedema—what are the significant predictors and how they affect the severity of lymphoedema? Breast J. 2006;12(6):536–43.
Aitken R, Gayes M, Rodger A, Chattey U, Forrest A. Arm morbidity within a trial of mastectomy and either node sample with selective radiotherapy or axillary clearance. BJS. 1989;76:568–71.
Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphoedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16(7):1959–72.
Villarini A, Pasanisi P, Raimondi M, Gargano G, Bruno E, Morelli D, et al. Preventing weight gain during adjuvant chemotherapy for breast cancer: a dietary intervention study. Breast Cancer Res Treat. 2012;135:581–9.
Sestak I, Harvie M, Howell A, Forbes JF, Dowsett M, Cuzick J. Weight change associated with Anastrozole and tamoxifen treatment in postmenopausal women with or at high risk of developing breast cancer. Breast Cancer Res Treat. 2012;134:727–37.
Kopec JA, Colangelo LH, Land SR, Julian TB, Brown AM, Anderson SJ, et al. Relationship between arm morbidity and patient-reported outcomes following surgery in women with node-negative breast cancer: NSABP protocol B-32. J Support Oncol. 2013;11(1):22–30.
Acknowledgement
Funded by Grants from the Australian National Health and Medical Research Council (NHMRC, 1046018, 512378, 1037786), the National Breast Cancer Foundation, the Australian Department of Health and Ageing, MBF Australia, and the Scottwood Trust, New Zealand. Rhana Pike, from the NHMRC Clinical Trials Centre, assisted with the manuscript. Funded by the Australian National Health and Medical Research Council, the National Breast Cancer Foundation, the Australian Department of Health and Ageing, MBF Australia, and the Scottwood Trust.
Disclosure
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wetzig, N., Gill, P.G., Espinoza, D. et al. Sentinel-Lymph-Node-Based Management or Routine Axillary Clearance? Five-Year Outcomes of the RACS Sentinel Node Biopsy Versus Axillary Clearance (SNAC) 1 Trial: Assessment and Incidence of True Lymphedema. Ann Surg Oncol 24, 1064–1070 (2017). https://doi.org/10.1245/s10434-016-5669-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5669-2