Abstract
Background
In human epidermal growth factor 2-positive breast cancer (HER2+BC), neoadjuvant chemotherapy and anti-HER2-targeted therapy (nCT) achieves a complete pathologic response (pCR) in 40–67% of patients. Posttreatment magnetic resonance imaging (pMRI) is considered the gold standard, with high specificity but lower sensitivity for assessing response. The authors previously determined that anti-HER2Th1 immune response is associated with pathologic response after nCT in HER2+BC patients. This study contrasted pMRI with anti-HER2Th1 response for assessing pCR in HER2+BC.
Methods
A retrospective review of HER2+BC patients at the authors’ institution was performed. Original pMRI reports were collected, and images were reviewed by a breast radiologist blinded to pCR and immune response. The post-nCT imaging-based tumor response was assessed by Response Evaluation Criteria in Solid Tumors. The anti-HER2Th1 response was determined by ex vivo stimulation of peripheral blood mononuclear cells with six major histocompatibility complex (MHC) class 2-derived HER2 peptides via enzyme-linked immunospot (ELISPOT). Posttreatment MRI and anti-HER2Th1 responses were cross-tabulated with pCR. Standard diagnostic metrics were computed.
Results
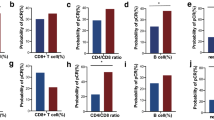
For 30 patients, pMRI and anti-HER2Th1 immune response were measured, with 13 patients (43.3%) achieving pCR. The mean anti-HER2Th1 response in pCR was 167 (range 53–418), and <pCR was 24 (range 0.4–53). The distributions were nearly non-overlapping. The anti-HER2Th1 response was superior to the original pMRI and had higher accuracy than the blinded pMRI review (area under the curve 0.97 vs 0.55; sensitivity 100 vs 46.2%; specificity 94.1 vs 64.7%; overall accuracy 96.7 vs 56.7%).
Conclusion
The presence of a high anti-HER2Th1 response is superior to pMRI for the assessment of pCR in HER2+BC. This assay has considerable promise, and validation in a large-scale study is warranted.



Similar content being viewed by others
References
Kaufmann M, von Minckwitz G, Rody A. Preoperative (neoadjuvant) systemic treatment of breast cancer. Breast. 2005;14(6):576–81.
Rastogi P, Anderson SL, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al., Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85.
Gralow JR, Burstein H, Wood W, et al, Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26:814–9.
Mieog S, van der Hage J, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007;18(2):CD005002.
Wolff AC, Davidson NE. Preoperative therapy in breast cancer: lessons from the treatment of locally advanced disease. Oncologist. 2002;7:239–45.
Untch M, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–7.
Broglio KR, Quintana M, Foster M, Olinger M, McGlothlin A, Berry SM, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016. doi:10.1001/jamaoncol.2015.6113.
Buzdar AU, Ibrahim N, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85.
Rahbar H, L.C. Rethinking preoperative breast magnetic resonance imaging. JAMA Oncol. 2015;1:1226–7.
Marinovich ML, Houssami N, Macaskill P, Sardanelli F, Irwig L, Mamounas EP, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. 2013;105:321–33.
DeMartini W, Lehman C, Partridge S. Breast MRI for cancer detection and characterization: a review of evidence-based clinical applications. Acad Radiol. 2008;15:408–16.
Schaefgen B, Mati M, Sinn HP, Golatta M, Stieber A, Rauch G, et al., Can routine imaging after neoadjuvant chemotherapy in breast cancer predict pathologic complete response? Ann Surg Oncol. 2016;23:789–95.
Datta J, et al. Anti-HER2 CD4(+) T-helper type 1 response is a novel immune correlate to pathologic response following neoadjuvant therapy in HER2-positive breast cancer. Breast Cancer Res. 2015;17:71.
Datta J, et al. Progressive loss of anti-HER2 CD4+T-helper type 1 response in breast tumorigenesis and the potential for immune restoration. Oncoimmunology. 2015;4:e1022301.
Datta J, et al. Association of depressed anti-HER2 T-helper type 1 response with recurrence in patients with completely treated HER2-positive breast cancer: role for immune monitoring. JAMA Oncol. 2016;2:242–6.
Eisenhauer EA, Therasseb P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: revised REGIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Koski GK, et al. A novel dendritic cell-based immunization approach for the induction of durable Th1-polarized anti-HER-2/neu responses in women with early breast cancer. J Immunother. 2012;35:54–65.
Hylton NM, Blume J, Bernreuter WK, Pisano ED, Rosen MA, Morris EA, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy: results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263:663–72.
Abraham DC, Jones R, Jones SE, Cheek JH, Peters GN, Knox SM, et al. Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer. 1996;78:91–100.
Yeh E, Slanetz P, Kopans DB, Rafferty E, Georgian-Smith D, Moy L, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184:868–77.
Schott AF, Roubidoux M, Helvie MA, et al., Clinical and radiological assessments to predict breast cancer pathologic complete response to neoadjuvant chemotherapy. Breast Cancer Res. 2005;92:231–8.
Chagpar AB, Middleton L, Sahin AA, Dempsey P, Buzdar AU, Mirza AN, et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg. 2006;243:257–64.
Chen JH, Feig B, Agrawal G, Yu H, Carpenter PM, Mehta RS, et al. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer. 2008;112:17–26.
Acknowledgments
This study was supported by National Institutes of Health R01 CA096997, Pennies in Action (www.penniesinaction.org), Lucy M. De La Cruz and Nadia Nocera—Henle Fund Research Fellowship, and University of Pennsylvania P30-CA016520.
Disclosure
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De La Cruz, L.M., McDonald, E.S., Mick, R. et al. Anti-HER2 CD4+ T-Helper Type 1 Immune Response is Superior to Breast MRI for Assessing Response to Neoadjuvant Therapy in Patients with HER2-Positive Breast Cancer. Ann Surg Oncol 24, 1057–1063 (2017). https://doi.org/10.1245/s10434-016-5651-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5651-z