Abstract
Background
Approximately 8–17 % of patients with von Hippel–Lindau (VHL) syndrome develop pancreatic neuroendocrine tumors (PNETs), with 11–20 % developing metastases. Tumor grade is predictive of prognosis.
Objective
The aim of this study was to determine if preoperative metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were associated with metastatic disease and tumor grade.
Methods
Sixty-two patients with VHL-associated PNETs prospectively underwent 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT). MTV, TLG, and maximum standardized uptake value (SUVmax) were measured using a semi-automatic method. Surgically resected PNETs were classified according to 2010 World Health Organization tumor grade classification. MTV, TLG, and SUVmax were analyzed by metastatic disease and tumor grade using the Mann–Whitney test.
Results
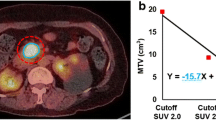
A total of 88 PNETs were identified by CT and 18F-FDG PET/CT, 10 of which were non-FDG-avid. Histologic grading was available for 20 surgical patients. Patients with metastatic PNETs had a higher TLG (median 25.9 vs. 7.7 mean SUV [SUVmean]*mL; p = 0.0092) compared with patients without metastasis, while patients with grade 2 PNETs had a higher MTV (median 6.9 vs. 2.6 mL; p = 0.034) and TLG (median 41.2 vs. 13.1 SUVmean*mL; p = 0.0035) compared with patients with grade 1 PNETs. No difference in tumor size or SUVmax was observed between the groups.
Conclusions
Patients with metastatic PNETs have a higher TLG compared with patients without metastasis. Grade 2 PNETs have a higher MTV and TLG compared with grade 1 PNETs. Tumor size and SUVmax were not associated with grade. Volumetric parameters on 18F-FDG PET/CT may be useful in detecting higher grade PNETs with a higher malignant potential that may need surgical intervention.



Similar content being viewed by others
References
Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361(9374):2059–2067.
Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320.
Hammel PR, Vilgrain V, Terris B, et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d’Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119(4):1087–1095.
Hough DM, Stephens DH, Johnson CD, Binkovitz LA. Pancreatic lesions in von Hippel-Lindau disease: prevalence, clinical significance, and CT findings. AJR Am J Roentgenol. 1994;162(5):1091–1094.
Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995;194(3):629–642.
Charlesworth M, Verbeke CS, Falk GA, Walsh M, Smith AM, Morris-Stiff G. Pancreatic lesions in von Hippel-Lindau disease? A systematic review and meta-synthesis of the literature. J Gastrointest Surg. 2012;16(7):1422–1428.
Yamasaki I, Nishimori I, Ashida S, Kohsaki T, Onishi S, Shuin T. Clinical characteristics of pancreatic neuroendocrine tumors in Japanese patients with von Hippel-Lindau disease. Pancreas. 2006;33(4):382–385.
Tamura K, Nishimori I, Ito T, Yamasaki I, Igarashi H, Shuin T. Diagnosis and management of pancreatic neuroendocrine tumor in von Hippel-Lindau disease. World J Gastroenterol. 2010;16(36):4515–4518.
Libutti SK, Choyke PL, Bartlett DL, et al. Pancreatic neuroendocrine tumors associated with von Hippel Lindau disease: diagnostic and management recommendations. Surgery. 1998;124(6):1153–1159.
Blansfield JA, Choyke L, Morita SY, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery. 2007;142(6):814-818; discussion 818 e811–812.
Morin E, Cheng S, Mete O, et al. Hormone profiling, WHO 2010 grading, and AJCC/UICC staging in pancreatic neuroendocrine tumor behavior. Cancer Med. 2013;2(5):701–711.
Strosberg J, Nasir A, Coppola D, Wick M, Kvols L. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40(9):1262–1268.
Strosberg JR, Cheema A, Weber J, Han G, Coppola D, Kvols LK. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol. 2011;29(22):3044–3049.
Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23(6):824–833.
Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20(11):2633–2642.
Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39(6):735–752.
Hasegawa T, Yamao K, Hijioka S, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46(1):32–38.
Yang M, Zeng L, Zhang Y, et al. TNM staging of pancreatic neuroendocrine tumors: an observational analysis and comparison by both AJCC and ENETS systems from 1 single institution. Medicine. 2015;94(12):e660.
Goldin SB, Bradner MW, Zervos EE, Rosemurgy AS 2nd. Assessment of pancreatic neoplasms: review of biopsy techniques. J Gastrointest Surg. 2007;11(6):783–790.
Lee JW, Kang CM, Choi HJ, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med. 2014;55(6):898–904.
Park SY, Cho A, Yu WS, et al. Prognostic value of total lesion glycolysis by 18F-FDG PET/CT in surgically resected stage IA non-small cell lung cancer. J Nucl Med. 2015;56(1):45–49.
Pak K, Cheon GJ, Nam HY, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55(6):884–890.
Lee JW, Cho A, Lee JH, et al. The role of metabolic tumor volume and total lesion glycolysis on (1)(8)F-FDG PET/CT in the prognosis of epithelial ovarian cancer. Eur J Nucl Med Mol Imaging. 2014;41(10):1898–1906.
Delbeke D, Martin WH. Positron emission tomography imaging in oncology. Radiol Clin North Am. 2001;39(5):883–917.
Bombardieri E, Aktolun C, Baum RP, et al. FDG-PET: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2003;30(12):B115–B124.
Masui T, Doi R, Ito T, et al. Diagnostic value of (18)F-fluorodeoxyglucose positron emission tomography for pancreatic neuroendocrine tumors with reference to the World Health Organization classification. Oncol Lett. 2010;1(1):155–159.
Pasquali C, Rubello D, Sperti C, et al. Neuroendocrine tumor imaging: can 18F-fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J Surg. 1998;22(6):588–592.
Larson SM, Erdi Y, Akhurst T, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2(3):159–171.
Fonti R, Larobina M, Del Vecchio S, et al. Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med. 2012;53(12):1829–1835.
Gimm O, DeMicco C, Perren A, Giammarile F, Walz MK, Brunaud L. Malignant pheochromocytomas and paragangliomas: a diagnostic challenge. Langenbecks Arch Surg. 2012;397(2):155–177.
Dholakia AS, Chaudhry M, Leal JP, et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89(3):539–546.
Satoh Y, Onishi H, Nambu A, Araki T. Volume-based parameters measured by using FDG PET/CT in patients with stage I NSCLC treated with stereotactic body radiation therapy: prognostic value. Radiology. 2014;270(1):275–281.
Moon SH, Choi JY, Lee HJ, et al. Prognostic value of 18F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: comparisons of volume-based metabolic parameters. Head Neck. 2013;35(1):15–22.
Schwartz M. A biomathematical approach to clinical tumor growth. Cancer. 1961;14:1272–1294.
Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123.
Pape UF, Jann H, Muller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113(2):256–265.
Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104(10):764–777.
Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35(6):853–860.
Alexiev BA, Darwin PE, Goloubeva O, Ioffe OB. Proliferative rate in endoscopic ultrasound fine-needle aspiration of pancreatic endocrine tumors: correlation with clinical behavior. Cancer. 2009;117(1):40–45.
Weynand B, Borbath I, Bernard V, et al. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: high reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology. 2014;25(6):389–395.
Tomimaru Y, Eguchi H, Tatsumi M, et al. Clinical utility of 2-[(18)F] fluoro-2-deoxy-d-glucose positron emission tomography in predicting World Health Organization grade in pancreatic neuroendocrine tumors. Surgery. 2015;157(2):269–276.
Kim HS, Choi JY, Choi DW, et al. Prognostic value of volume-based metabolic parameters measured by (18)F-FDG PET/CT of pancreatic neuroendocrine tumors. Nucl Med Mol Imaging. 2014;48(3):180–186.
Ichikawa T, Peterson MS, Federle MP, et al. Islet cell tumor of the pancreas: biphasic CT versus MR imaging in tumor detection. Radiology. 2000;216(1):163–171.
Viola KV, Sosa JA. Current advances in the diagnosis and treatment of pancreatic endocrine tumors. Curr Opin Oncol. 2005;17(1):24–27.
Sadowski SM, Weisbrod AB, Ellis R, et al. Prospective evaluation of the clinical utility of 18-fluorodeoxyglucose PET CT scanning in patients with von hippel-lindau-associated pancreatic lesions. J Am Coll Surg. 2014;218(5):997–1003.
Funding
This research was made possible through the NIH Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Newport Foundation, The American Association for Dental Research, The Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program.
Disclosures
Kei Satoh, Samira M. Sadowski, William Dieckmann, Martha Quezado, Naris Nilubol, Electron Kebebew, and Dhaval Patel have no conflicts of interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satoh, K., Sadowski, S.M., Dieckmann, W. et al. 18F-FDG PET/CT Volumetric Parameters are Associated with Tumor Grade and Metastasis in Pancreatic Neuroendocrine Tumors in von Hippel–Lindau Disease. Ann Surg Oncol 23 (Suppl 5), 714–721 (2016). https://doi.org/10.1245/s10434-016-5541-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5541-4