Abstract
Background
Pathologic complete response (pCR) after neoadjuvant chemotherapy (NAC) can be used as an independent prognostic factor in neoadjuvant trials. The objective of this study was to determine the impact of Ki 67 expression and site of response on overall survival (OS) and disease-free survival (DFS) across different molecular subtypes of breast cancer following NAC.
Methods
Records from 357 patients who received NAC from 2004 to 2011 were reviewed. Univariate and multivariate analyses were performed to analyze clinical and pathological factors that influence pCR and DFS.
Results
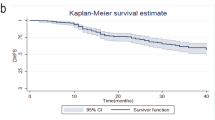
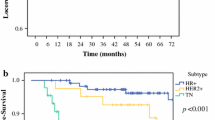
Mean follow-up time was 45 months (range 12–112). pCR was achieved in 82 patients (23 %). According to molecular subtypes, rates of pCR were significantly higher for patients with HER2-positive and triple-negative tumors (69.4 and 32.7 %, respectively; p < 0.001) compared with other molecular subtypes. pCR was a predictive factor of longer OS and DFS. The hazard ratio for DFS in patients with positive lymph nodes (ypN1) after NAC was 2.48 (95 % confidence interval 1.47–4.19). Multivariate analysis showed that molecular subtype, changes in Ki 67 expression, and axillary lymph node response were significantly predictors of OS and DFS.
Conclusions
pCR in the axilla and posttreatment changes in Ki 67 after NAC are associated with improved survival. Depending on axillary staging before NAC, detection of minimal residual disease—defined as the presence of isolated tumor cells in the SLN after NAC—may confer different prognosis. Further studies are needed to tailor treatments for patients with residual disease after NAC.


Similar content being viewed by others
References
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr. 2001;(30):96–102.
Bear HD. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from national surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol. 2003;21(22):4165–74.
Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–85.
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224–37.
Tiezzi DG, Andrade JM, Marana HRC, Zola FE, Peria FM. Breast conserving surgery after neoadjuvant therapy for large primary breast cancer. Eur J Surg Oncol. 2008;34(8):863–7.
Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–9.
Shin H-C, Han W, Moon H-G, et al. Breast-conserving surgery after tumor downstaging by neoadjuvant chemotherapy is oncologically safe for stage III breast cancer patients. Ann Surg Oncol. 2013;20(8):2582–9.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Corben AD, Abi-Raad R, Popa I, et al. Pathologic response and long-term follow-up in breast cancer patients treated with neoadjuvant chemotherapy: a comparison between classifications and their practical application. Arch Pathol Lab Med. 2013;137(8):1074–82.
Minckwitz von G, Untch M, Blohmer J-U, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804.
Aebi S, Gelber S, Anderson SJ, et al. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomised trial. Lancet Oncol. 2014;15(2):156–63.
Kuerer HM, Sahin AA, Hunt KK, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230(1):72–8.
Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomized, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–40.
Rouzier R, Extra J-M, Klijanienko J, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002;20(5):1304–10.
Zhang GC, Zhang YF, Xu FP, et al. Axillary lymph node status, adjusted for pathologic complete response in breast and axilla after neoadjuvant chemotherapy, predicts differential disease-free survival in breast cancer. Curr Oncol. 2013;20(3):e180–92.
von Minckwitz G, Schmitt WD, Loibl S, et al. Ki 67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin Cancer Res. 2013;19(16):4521–31.
Montagna E, Bagnardi V, Viale G, et al. Changes in PgR and Ki67 in residual tumour and outcome of breast cancer patients treated with neoadjuvant chemotherapy. Ann Oncol. 2015;26(2):307–13.
Edge SB, Compton CC, Fritz AG, et al. American joint committee on cancer (AJCC) cancer staging manual. 7th ed. Chicago: Springer; 2010.
Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann oncol. 2011;22:1736–47.
Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in breast cancer working group. J Natl Cancer Inst. 2011;103(22):1656–64.
Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22(12):2303–12.
Feldman LD, Hortobagyi GN, Buzdar AU, Ames FC, Blumenschein GR. Pathological assessment of response to induction chemotherapy in breast cancer. Cancer Res. 1986;46(5):2578–81.
Jones RL, Lakhani SR, Ring AE, Ashley S, Walsh G, Smith IE. Pathological complete response and residual DCIS following neoadjuvant chemotherapy for breast carcinoma. Br J Cancer. 2006;94(3):358–62.
Mazouni C, Peintinger F, Wan-Kau S, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007;25(19):2650–5.
Minckwitz von G, Untch M, Nüesch E, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2010;125(1):145–56.
Mougalian SS, Hernandez M, Xiudong L, et al. Outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastasis and pathologic complete response after primary systemic chemotherapy. JAMA Oncol. 2015;30:1–9.
Straver ME, Rutgers EJT, Rodenhuis S, et al. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 2010;17(9):2411–8.
Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81.
Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29(25):3351–7.
de Azambuja E, Cardoso F, de Castro G Jr, et al. Ki67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12.155 patients. Br J Cancer. 2007;96(10):1504–13.
Matsubara N, Mukai H, Fujii S, Wada M. Different prognostic significance of Ki-67 change between pre- and post-neoadjuvant chemotherapy in various subtypes of breast cancer. Breast Cancer Res Treat 2013;137:203–12.
Yamazaki N, Wada N, Yamauchi C, Yoneyama K. High expression of post-treatment Ki-67 status is a risk factor for locoregional recurrence following breast- conserving surgery after neoadjuvant chemotherapy. EJSO. 2015;41:617–24.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72.
Dominici LS, Negron Gonzalez VM, Buzdar AU, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer. 2010;116(12):2884–9.
Boileau J-F, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64.
Hunt KK, Ballman KV, McCall LM, et al. Factors associated with local-regional recurrence after a negative sentinel node dissection: results of the ACOSOG Z0010 trial. Ann Surg. 2012;256(3):428–36.
Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–8.
Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27(15):2466–73.
Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonié Bordeaux Groupe Sein (IBBGS). Ann Oncol. 1999;10(1):47–52.
Mittendorf EA, Buchholz TA, Tucker SL, et al. Impact of chemotherapy sequencing on local-regional failure risk in breast cancer patients undergoing breast-conserving therapy. Ann Surg. 2013;257(2):173–9.
Cebrecos I, Córdoba O, Deu J, Xercavins J, Rubio IT. Can we predict local recurrence in breast conserving surgery after neoadjuvant chemotherapy? EJSO. 2010;36(6):528–34.
Disclosure
None of the authors have financial relationship to disclosure.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diaz-Botero, S., Espinosa-Bravo, M., Gonçalves, V.R. et al. Different Prognostic Implications of Residual Disease After Neoadjuvant Treatment: Impact of Ki 67 and Site of Response. Ann Surg Oncol 23, 3831–3837 (2016). https://doi.org/10.1245/s10434-016-5339-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5339-4