Abstract
Purpose
Published data have shown heterogeneous outcomes for high-risk prostate cancer. Thus, we tried to identify more precise risk stratification system for contemporary high-risk prostate cancer.
Methods
Classifying patients according to National Comprehensive Cancer Network risk groups, we reviewed data of 1,905 men who underwent radical prostatectomy (RP) at our institution from 2006 to 2013. For our analyses, high-risk prostate cancers meeting at least one of two following factors were categorized as unfavorable high-risk prostate cancer: biopsy primary Gleason pattern 5 and/or multiple (≥2) high-risk criteria present. All other men with high-risk prostate cancer were designated as having favorable high-risk disease. Postoperative outcomes, including biochemical recurrence-free survivals were assessed and compared via log-rank test and Cox proportional hazards model.
Results
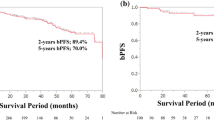
In multivariable analysis, primary Gleason 5 pattern on biopsy (p = 0.008) and multiple (≥2) high-risk criteria (p < 0.001) were observed to be independent predictors of the risk of biochemical recurrence amongst high-risk group undergoing RP. Favorable high-risk prostate cancer group showed a significantly higher 5-year biochemical recurrence-free survival than unfavorable high-risk group (56.35 vs. 18.75 %; log-rank test: p < 0.001). Favorable high-risk group demonstrated significantly lower 5-year biochemical recurrence-free survival than intermediate-risk group (56.07 vs. 82.05 %; log-rank test: p < 0.001).
Conclusions
A significant heterogeneity existed in biochemical outcomes of contemporary patients with high-risk prostate cancer who underwent definitive RP. According to primary Gleason pattern and number of high-risk criteria present, high-risk group should be stratified further into favorable and unfavorable disease.

Similar content being viewed by others
References
NCCN. Clinical Practice Guidelines in Oncology (NCCN Guideline®). Prostate cancer v4.2013. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Bolla M, van Poppel H, Tombal B, et al; European Organisation for Research and Treatment of Cancer, Radiation Oncology and Genito-Urinary Groups. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380:2018–27.
Swanson GP, Hussey MA, Tangen CM, et al; SWOG 8794. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol. 2007;25:2225–9.
Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–30.
Messing EM, Manola J, Yao J, et al. Eastern Cooperative Oncology Group study EST 3886. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–9.
Dorff TB, Flaig TW, Tangen CM, et al. Adjuvant androgen deprivation for high-risk prostate cancer after radical prostatectomy: SWOG S9921 study. J Clin Oncol. 2011;29:2040–5.
van den Ouden D, Hop WC, Schröder FH. Progression in and survival of patients with locally advanced prostate cancer (T3) treated with radical prostatectomy as monotherapy. J Urol. 1998;160:1392–7.
Van Poppel H, Goethuys H, Callewaert P, Vanuytsel L, Van de Voorde W, Baert L. Radical prostatectomy can provide a cure for well-selected clinical stage T3 prostate cancer. Eur Urol. 2000;38:372–9.
Carver BS, Bianco FJ Jr, Scardino PT, Eastham JA. Long-term outcome following radical prostatectomy in men with clinical stage T3 prostate cancer. J Urol. 2006;176:564–8.
Yossepowitch O, Eggener SE, Bianco FJ Jr, Carver BS, Serio A, Scardino PT, Eastham JA. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J Urol. 2007;178:493–9.
Sundi D, Wang VM, Pierorazio PM, et al. Very-high-risk localized prostate cancer: definition and outcomes. Prostate Cancer Prostatic Dis. 2014;17:57–63.
Reese AC, Pierorazio PM, Han M, Partin AW. Contemporary evaluation of the National Comprehensive Cancer Network prostate cancer risk classification system. Urology. 2012;80:1075–9.
Joniau S, Briganti A, Gontero P, et al. European Multicenter Prostate Cancer Clinical and Translational Research Group (EMPaCT). Stratification of high-risk prostate cancer into prognostic categories: a European multi-institutional study. Eur Urol. 2014. doi:10.1016/j.eururo.2014.01.020.
D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74.
Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–42.
Abdollah F, Karnes RJ, Suardi N, et al. Predicting survival of patients with node-positive prostate cancer following multimodal treatment. Eur Urol. 2014;65:554–62.
Spahn M, Joniau S, Gontero P, et al. Outcome predictors of radical prostatectomy in patients with prostate-specific antigen greater than 20 ng/ml: a European multi-institutional study of 712 patients. Eur Urol. 2010;58:1–7.
Walz J, Joniau S, Chun FK, et al. Pathological results and rates of treatment failure in high-risk prostate cancer patients after radical prostatectomy. BJU Int. 2011;107:765–70.
Hashine K, Yuasa A, Shinomori K, Shirato A, Ninomiya I, Teramoto N. Tertiary Gleason pattern 5 and oncological outcomes after radical prostatectomy. Jpn J Clin Oncol. 2011;41:571–6.
Trock BJ, Guo CC, Gonzalgo ML, Magheli A, Loeb S, Epstein JI. Tertiary Gleason patterns and biochemical recurrence after prostatectomy: proposal for a modified Gleason scoring system. J Urol. 2009;182:1364–70.
Stock RG, Cesaretti JA, Hall SJ, Stone NN. Outcomes for patients with high-grade prostate cancer treated with a combination of brachytherapy, external beam radiotherapy and hormonal therapy. BJU Int. 2009;104:1631–6.
Sabolch A, Feng FY, Daignault-Newton S, et al. Gleason pattern 5 is the greatest risk factor for clinical failure and death from prostate cancer after dose-escalated radiation therapy and hormonal ablation. Int J Radiat Oncol Biol Phys. 2011;81:e351–60.
Vis AN, Roemeling S, Kranse R, Schröder FH, van der Kwast TH. Should we replace the Gleason score with the amount of high-grade prostate cancer? Eur Urol. 2007;51:931–9.
Nanda A, Chen MH, Renshaw AA, D’Amico AV. Gleason Pattern 5 prostate cancer: further stratification of patients with high-risk disease and implications for future randomized trials. Int J Radiat Oncol Biol Phys. 2009;74:1419–23.
Cheng L, Koch MO, Juliar BE, Daggy JK, Foster RS, Bihrle R, Gardner TA. The combined percentage of Gleason patterns 4 and 5 is the best predictor of cancer progression after radical prostatectomy. J Clin Oncol. 2005;23:2911–7.
D’Ambrosio DJ, Hanlon AL, Al-Saleem T, et al. The proportion of prostate biopsy tissue with Gleason pattern 4 or 5 predicts for biochemical and clinical outcome after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:1082–7.
Gallina A, Chun FK, Suardi N, et al. Comparison of stage migration patterns between Europe and the USA: an analysis of 11 350 men treated with radical prostatectomy for prostate cancer. BJU Int. 2008;101:1513–8.
Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101:1280–3.
Disclosure
All authors have no conflict of interest with any institution or product.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jo, J.K., Kook, H.R., Byun, SS. et al. Stratification of Contemporary Patients Undergoing Radical Prostatectomy for High-risk Prostate Cancer. Ann Surg Oncol 22, 2088–2093 (2015). https://doi.org/10.1245/s10434-014-4183-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4183-7