Abstract
Introduction
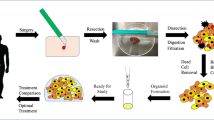
We have hypothesized that biofabrication of appendiceal tumor organoids allows for a more personalized clinical approach and facilitates research in a rare disease.
Methods
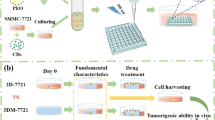
Appendiceal cancer specimens obtained during cytoreduction with hyperthermic intraperitoneal chemotherapy procedures (CRS/HIPEC) were dissociated and incorporated into an extracellular matrix-based hydrogel system as three-dimensional (3D), patient-specific tumor organoids. Cells were not sorted, preserving tumor heterogeneity, including stroma and immune cell components. Following establishment of organoid sets, chemotherapy drugs were screened in parallel. Live/dead staining and quantitative metabolism assays recorded which chemotherapies were most effective in killing cancer cells for a specific patient. Maintenance of cancer phenotypes were confirmed by using immunohistochemistry.
Results
Biospecimens from 12 patients were applied for organoid development between November 2016 and May 2018. Successful establishment rate of viable organoid sets was 75% (9/12). Average time from organoid development to chemotherapy testing was 7 days. These tumors included three high-grade appendiceal (HGA) and nine low-grade appendiceal (LGA) primaries obtained from sites of peritoneal metastasis. All tumor organoids were tested with chemotherapeutic agents exhibited responses that were either similar to the patient response or within the variability of the expected clinical response. More specifically, HGA tumor organoids derived from different patients demonstrated variable chemotherapy tumor-killing responses, whereas LGA organoids tested with the same regimens showed no response to chemotherapy. One LGA set of organoids was immune-enhanced with cells from a patient-matched lymph node to demonstrate feasibility of a symbiotic 3D reconstruction of a patient matched tumor and immune system component.
Conclusions
Development of 3D appendiceal tumor organoids is feasible even in low cellularity LGA tumors, allowing for individual patient tumors to remain viable for research and personalized drug screening.




Similar content being viewed by others
References
Mazzocchi AR, Rajan SAP, Votanopoulos KI, Hall AR, Skardal A. In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Sci Rep. 2018;8:2886.
Mazzocchi A, Soker S, Skardal A. Biofabrication technologies for developing in vitro tumor models. In: Soker S, Skardal A (eds). Tumor organoids. Cancer Drug Discovery and Development. Cham: Humana Press; 2018. pp. 51–70.
Skardal A, Devarasetty M, Forsythe S, Atala A, Soker SA. Reductionist metastasis-on-a-chip platform for in vitro tumor progression modeling and drug screening. Biotechnol Bioeng. 2016;113:2020–32.
Skardal A, Devarasetty M, Kang HW, Mead I, Bishop C, Shupe T, et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015;25:24–34.
Skardal A, Devarasetty M, Soker S, Hall AR. In situ patterned micro 3D liver constructs for parallel toxicology testing in a fluidic device. Biofabrication. 2015;7:031001.
Skardal A. Extracellular matrix-like hydrogels for applications in regenerative medicine. In: Connon CJ, Hamley IW (eds). Hydrogels in cell-based therapies. London: Royal Society of Chemistry; 2014.
Skardal A, Murphy SV, Crowell K, Mack D, Atala A, Soker S. A tunable hydrogel system for long-term release of cell-secreted cytokines and bioprinted in situ wound cell delivery. J Biomed Mater Res B Appl Biomater. 2016;105(7):1986–2000.
Skardal A, Smith L, Bharadwaj S, Atala A, Soker S, Zhang Y. Tissue specific synthetic ECM hydrogels for 3-D in vitro maintenance of hepatocyte function. Biomaterials. 2012;33:4565–75.
Skardal A, Zhang J, McCoard L, Xu X, Oottamasathien S, Prestwich GD. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng Part A. 2010;16:2675–85.
Skardal A, Zhang J, Prestwich GD. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31:6173–81.
Skardal A, Murphy SV, Devarasetty M, Mead I, Kang HW, Seol YJ, et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep. 2017;7:8837.
Devarasetty M, Skardal A, Cowdrick K, Marini F, Soker S. Bioengineered submucosal organoids for in vitro modeling of colorectal cancer. Tissue Eng Part A. 2017;23:1026–41.
Spaeth EL, Labaff AM, Toole BP, Klopp A, Andreeff M, Marini FC. Mesenchymal CD44 expression contributes to the acquisition of an activated fibroblast phenotype via TWIST activation in the tumor microenvironment. Cancer Res. 2013;73:5347–59.
Levine EA, Blazer DG, Kim MK, Shen P, Stewart JH, Guy C, et al. Gene expression profiling of peritoneal metastases from appendiceal and colon cancer demonstrates unique biologic signatures and predicts patient outcomes. J Am Coll Surg. 2012;214:599–606 (discussion 606–7).
Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920–6.
Votanopoulos KI, Russell G, Randle RW, Shen P, Stewart JH, Levine EA. Peritoneal surface disease (PSD) from appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): overview of 481 cases. Ann Surg Oncol. 2015;22:1274–9.
Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013.
Acknowledgement
The authors thank Libby McWilliams (Procurement Manager), Kathleen Cummings (Protocol and Data Manager) and the Wake Forest Advanced Tumor Bank Shared Resource.
Funding
AS acknowledges funding through the Wake Forest Clinical and Translational Science Institute Open Pilot Program. AS and KV acknowledge funding through the Comprehensive Cancer Center at Wake Forest Baptist Medical Center’s Clinical Research Associate Director Pilot Funds.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
The authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Votanopoulos, K.I., Mazzocchi, A., Sivakumar, H. et al. Appendiceal Cancer Patient-Specific Tumor Organoid Model for Predicting Chemotherapy Efficacy Prior to Initiation of Treatment: A Feasibility Study. Ann Surg Oncol 26, 139–147 (2019). https://doi.org/10.1245/s10434-018-7008-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-7008-2