Abstract
Background
Failure to rescue (FTR) is a quality-of-care indicator in pancreatic surgery, but may also identify patients who may not tolerate major postoperative complications despite being treated with best available care. Previous studies found that high visceral adipose tissue-to-skeletal muscle ratio is associated with poor outcomes following pancreaticoduodenectomy (PD). The aim of the study is to assess the impact of sarcopenic obesity on occurrence of FTR from major complications in cancer patients undergoing PD.
Methods
Prospectively collected data from three high-volume hospitals were reviewed. Total abdominal muscle area (TAMA) and visceral fat area (VFA) were assessed at preoperative staging computed tomography scan. Sarcopenic obesity was defined as high VFA/TAMA ratio. FTR was defined as postoperative mortality following major complication.
Results
120 patients with major complications were included. FTR occurred in 23 (19.2%) patients. The “seminal” complications leading to FTR were pancreatic or biliary fistula-related sepsis (n = 14), postoperative pancreatic fistula (POPF)-related hemorrhage (n = 5), and duodenojejunal anastomosis leak-related sepsis (n = 1). On univariate analysis, older age [odds ratio (OR) 3.5, p = 0.034], American Society of Anesthesiologists (ASA) score 3+ (OR 4.2, p = 0.005), cardiovascular disease (OR 3.3, p = 0.013), low serum albumin (OR 2.6, p = 0.042), sarcopenic obesity (OR 4.2, p = 0.009), POPF (OR 3.1, p = 0.027), and cardiorespiratory complications (OR 3.7, p = 0.011) were significantly associated with FTR. On multivariate analysis, sarcopenic obesity [OR 5.7, 95% confidence interval (CI) 1.6–20.7, p = 0.008], ASA score 3+ (OR 4.1, 95% CI 1.2–14.3, p = 0.025), and pancreatic fistula (OR 3.2, 95% CI 1.0–10.2, p = 0.045) were independently associated with FTR.
Conclusion
Sarcopenic obesity, low preoperative physical status, and occurrence of pancreatic fistula are associated with significantly higher risk of FTR from major complications after PD.

Similar content being viewed by others
References
Gooiker GA, van Gijn W, Wouters MW, et al. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 2011;98(4):485–494.
Balzano G, Capretti G, Callea G, Cantu E, Carle F, Pezzilli R. Overuse of surgery in patients with pancreatic cancer. A nationwide analysis in Italy. HPB (Oxford). 2016;18(5):470–478.
Uzunoglu FG, Reeh M, Vettorazzi E, et al. Preoperative pancreatic resection (PREPARE) score: a prospective multicenter-based morbidity risk score. Ann Surg. 2014;260(5):857–863; discussion 863–854.
Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10(9):1199–1210; discussion 1210–1191.
Silber JH, Romano PS, Rosen AK, Wang Y, Even-Shoshan O, Volpp KG. Failure-to-rescue: comparing definitions to measure quality of care. Med Care. 2007;45(10):918–925.
Ghaferi AA, Dimick JB. Importance of teamwork, communication and culture on failure-to-rescue in the elderly. Br J Surg. 2016;103(2):e47–51.
Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–2276.
Belyaev O, Herzog T, Kaya G, et al. Pancreatic surgery in the very old: face to face with a challenge of the near future. World J Surg. 2013;37(5):1013–1020.
Englesbe MJ, Lee JS, He K, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256(2):255–261.
Pecorelli N, Carrara G, De Cobelli F, et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg. 2016;103(4):434–442.
Sandini M, Bernasconi DP, Fior D, et al. A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition (Burbank, Los Angeles County, Calif.). 2016;32(11–12):1231–1237.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457.
Balzano G, Zerbi A, Capretti G, Rocchetti S, Capitanio V, Di Carlo V. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg. 2008;95(3):357–362.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006.
Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–2338.
Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–1547.
Pecorelli N, Balzano G, Capretti G, Zerbi A, Di Carlo V, Braga M. Effect of surgeon volume on outcome following pancreaticoduodenectomy in a high-volume hospital. J Gastrointest Surg. 2012;16(3):518–523.
Gianotti L, Braga M, Gentilini O, Balzano G, Zerbi A, Di Carlo V. Artificial nutrition after pancreaticoduodenectomy. Pancreas. 2000;21(4):344–351.
Mise Y, Vauthey JN, Zimmitti G, et al. Ninety-day postoperative mortality is a legitimate measure of hepatopancreatobiliary surgical quality. Ann Surg. 2015;262(6):1071.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13.
Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20–25.
Williams BA, Mandrekar JN, Mandrekar SJ, Cha SS, Furth AF. Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes.: Rochester, MN, Mayo Foundation, Technical Report Series 79;2006.
Glantz SA, Slinker BK. Primer of applied regression and analysis of variance. New York: McGraw-Hill, Health Professions Division; 1990.
Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–1748.
Joseph B, Morton JM, Hernandez-Boussard T, Rubinfeld I, Faraj C, Velanovich V. Relationship between hospital volume, system clinical resources, and mortality in pancreatic resection. J Am Coll Surg. 2009;208(4):520–527.
Sheetz KH, Dimick JB, Ghaferi AA. Impact of hospital characteristics on failure to rescue following major surgery. Ann Surg. 2016;263(4):692–697.
Hasselager R, Gogenur I. Core muscle size assessed by perioperative abdominal CT scan is related to mortality, postoperative complications, and hospitalization after major abdominal surgery: a systematic review. Langenbeck Arch Surg. 2014;399(3):287–295.
Ruiz M, Cefalu C, Reske T. Frailty syndrome in geriatric medicine. Am J Med Sci. 2012;344(5):395–398.
Kaplan SJ, Pham TN, Arbabi S, et al. Association of radiologic indicators of frailty with 1-year mortality in older trauma patients: opportunistic screening for sarcopenia and osteopenia. JAMA Surg. 2017;152(2):e164604.
Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887.
Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab. 2010;95(9):4338–4344.
Pedersen BK. The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol. 2009;587(Pt 23):5559–5568.
Braga M, Capretti G, Pecorelli N, et al. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg. 2011;254(5):702–708; discussion 707–708.
Tamirisa NP, Parmar AD, Vargas GM, et al. Relative contributions of complications and failure to rescue on mortality in older patients undergoing pancreatectomy. Ann Surg. 2016;263(2):385–391.
Kirihara Y, Takahashi N, Hashimoto Y, et al. Prediction of pancreatic anastomotic failure after pancreatoduodenectomy: the use of preoperative, quantitative computed tomography to measure remnant pancreatic volume and body composition. Ann Surg. 2013;257(3):512–519.
Sandini M, Bernasconi DP, Ippolito D, et al. Preoperative computed tomography to predict and stratify the risk of severe pancreatic fistula after pancreatoduodenectomy. Medicine (Baltimore). 2015;94(31):e1152.
Barberan-Garcia A, Ubre M, Roca J, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg. 2017. https://doi.org/10.1097/SLA.0000000000002293.
Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27(4):1072–1082.
Glance LG, Osler TM, Neuman MD. Redesigning surgical decision making for high-risk patients. N Engl J Med. 2014;370(15):1379–1381.
Sutton JM, Abbott DE. Neoadjuvant therapy for pancreas cancer: past lessons and future therapies. World J Gastroenterol. 2014;20(42):15564–15579.
West MA, Loughney L, Lythgoe D, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesthesia. 2015;114(2):244–251.
Disclosure
The authors disclose no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 8365 kb)
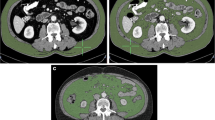
Supplementary Material 1. CT scan at the third lumbar vertebra level in a sarcopenic obese male patient included in the study. (a) unprocessed imaging; (b) processed imaging. Subcutaneous fat area (SFA) is highlighted in yellow, total abdominal muscle area (TAMA) in red, and visceral fat area (VFA) in green. SFA was 299 cm2, TAMA 33 cm2/m2, VFA 165 cm2. His VFA/TAMA ratio was 5.0.
Supplementary material 2 (DOCX 107 kb)
Supplementary Material 2. Definition of postoperative complications used in the study.
Supplementary material 3 (TIFF 3939 kb)
Supplementary Material 3. Receiver operating characteristic curve of final multivariate model for failure to rescue.
Supplementary material 4 (DOCX 21 kb)
Supplementary Material 4. Univariate and multivariate logistic regression analysis of potential predictors associated with failure to rescue after pancreaticoduodenectomy, entering visceral fat area instead of visceral adipose tissue-to-skeletal muscle ratio in the multivariate model.
Rights and permissions
About this article
Cite this article
Pecorelli, N., Capretti, G., Sandini, M. et al. Impact of Sarcopenic Obesity on Failure to Rescue from Major Complications Following Pancreaticoduodenectomy for Cancer: Results from a Multicenter Study. Ann Surg Oncol 25, 308–317 (2018). https://doi.org/10.1245/s10434-017-6216-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6216-5