Abstract
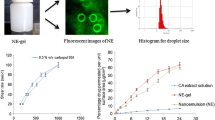
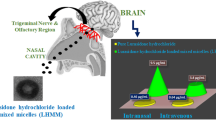
Alzheimer’s disease (AD) is a very common disorder that affects the elderly. There are relatively few medications that can be used orally or as a suspension to treat AD. A mucoadhesive (o/w) nano emulsion of mefenamic acid was made by adding Carbopol 940P to the optimised drug nanoemulsion using distilled water as the aqueous phase (6%); Solutol HS: tween 20 (3.6%) as the surfactant and co-surfactant; and clove oil: TPGS (0.4%) as the oil phase and mefenamic acid as the drug (2.8 mg/ml). The mucoadhesive nanoemulsion (S40.5%w/v) had a particle size of 91.20 nm, polydispersity index of 0.270, and surface charge of − 12.4 mV. Significantly higher (p < 0.001) drug release (89.37%) was observed for mucoadhesive drug formulation in comparison to mucoadhesive drug suspension (25.64%) at 8 h. The ex vivo nasal permeation of 83.03% in simulated nasal fluid and 85.71% in artificial cerebrospinal fluid was observed. The percent inhibition and inhibitory concentration (IC50) of mucoadhesive drug nanoemulsion were found to be 91.57 ± 2.69 and 6.76 respectively. Higher cell viability on glioblastoma cells (85–80%) was researched for mucoadhesive nanoemulsion as compared to drug suspension (80–70%). Significantly higher (p < 0.001) drug absorption and Cmax (491.94 ± 24.13 ng/ml) of mucoadhesive drug nanoemulsion were observed than mucoadhesive drug suspension (107.46 ± 11.46 ng/ml) at 8 h. The stability studies confirmed that the formulation was stable at 40°C ± 2°C and 75 ± 5% RH. The authors concluded that the mucoadhesive mefenamic acid–loaded nanoemulsion may be an effective technique for treating Alzheimer’s disease by intranasal route.
Graphical Abstract

















Similar content being viewed by others
References
Clark CM, Karlawish JH. Alzheimer disease: current concepts and emerging diagnostic and therapeutic strategies. Ann Intern Med. 2003;138:400–10.
Selkoe DJ. The origins of Alzheimer disease: a is for amyloid. JAMA. 2000;283:1615–7.
Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998;21:428–33.
Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O’Donnell M, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385(9967):549–62.
Cacquevel M, Lebeurrier N, Cheenne S, Vivien D. Cytokines in neuroinflammation and Alzheimer’s disease. Curr Drug Targets. 2004;5:529–34.
Yan Q, Zhang J, Liu H, Babu KS, Vassar R, Biere AL, et al. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J Neurosci. 2003;23:7504–9.
Sugaya K, Uz T, Kumar V, Manev H. New anti-inflammatory treatment strategy in Alzheimer’s disease. Jpn J Pharmacol. 2000;82:85–94.
McGeer EG, McGeer PL. Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;5:741–9.
Asanuma M, Nishibayashi AS, Miyazaki I, Kohno M, Ogawa N. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. J Neurochem. 2001;76:1895–904.
Joo Y, Kim HS, Woo RS, Park CH, Shin KY, Lee JP, et al. Mefenamic acid shows neuroprotective effects and improves cognitive impairment in in vitro and in vivo Alzheimer’s disease models. Mol Pharmacol. 2006;69(1):76–84.
Daniels MJ, Rivers-Auty J, Schilling T, Spencer NG, Watremez W, Fasolino V, et al. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat Commun. 2016;7:12504.
Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49.
Cabral SC. Syntesis and charactherization of celecoxib-loaded nanomicellar topical formulation on diabetic retinopathy. Universidade De Coimbra. 2017.
Boyd-Kimball D, Sultana R, Mohmmad-Abdul H, Butterfield DA. Rodent Aβ(1–42) exhibits oxidative stress properties similar to those of human Aβ(1–42): implications for proposed mechanisms of toxicity. J Alzheimers Dis. 2004;6:515–25.
Yatin SM, Varadarajan S, Butterfield DA. Vitamin E prevents nti-infl’s amyloid β-peptide(1–42)-induced neuronal protein oxidation and reactive oxygen species production. J Alzheimers Dis. 2000;2:123–31.
Bhavna MS, Ali M, Baboota S, Sahni JK, Bhatnagar A, Ali J. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev Ind Pharm. 2014;40:278–87.
Bonferoni MC, Rossi S, Sandri G, Ferrari F, Gavini E, Rassu G, et al. Nanoemulsions for “nose-to-brain” drug delivery. Pharmaceutics. 2019;11(2):84.
Jain K, Kumar SR, Sood S, Gowthamarajan S. Enhanced oral bioavailability of atorvastatin via oil-in-water nanoemulsion using aqueous titration method. J Pharm Sci Res. 2013;5(1):18–25.
Sharma S, Sahni JK, Ali J, Baboota S. Effect of high-pressure homogenization on formulation of TPGS loaded nanoemulsion of rutin – Pharmacodynamic and antioxidant studies. J Drug Deliv Sci Technol. 2014;22(4):1–11.
Sawatdee S, Atipairin A, Yoon AS, Srichana T, Changsan N, Suwandecha T. Formulation development of albendazole-loaded self-microemulsifying chewable tablets to enhance dissolution and bioavailability. Pharmaceutics. 2019;11(3):134.
Patel HC, Parmar G, Seth AK, Patel JD, Patel SR. Formulation and evaluation of o/w nanoemulsion of ketoconazole. Int J Pharmaceut Sci. 2013;4:338–51.
Sunitha S, Wankar J, Ajimera T. Design, developmentand evaluation of nanoemulsion and nanogel of itraconazole for transdermal delivery. J Sci Res Pharmacy. 2014;3(1):6–11.
Indora N, Kaushik D. Design, development and evaluation of ethosomal gel of fluconazole for topical fungal infection. Int J Eng Sci Invent Res Develop. 2015;1:280–306.
Baboota S, Rahman M, Kumar A, Sharma S, Sahni J, Ali J. Submicron size formulation of linseed oil containing omega-3 fatty acid for topical delivery. J Dispersion Sci Technol. 2012;33:1259–66.
Mitkari BV, Korde SA, Mahadik KR, Kokare CK. Formulation and evaluation of topical liposomal gel for fluconazole. Indian J Pharm Educ Res. 2010;44(4):324–33.
Imran M, Almehmadi M, Alsaiari AA, Kamal M, Alshammari MK, Alzahrani MO, et al. Intranasal delivery of a silymarin loaded microemulsion for the effective treatment of Parkinson’s disease in rats: formulation, optimization, characterization, and in vivo evaluation. Pharmaceutics. 2023;15:618.
Talegaonkar S, Mishra PR. Intranasal delivery: an approach to bypass the blood brain barrier. Indian J Pharmacol. 2004;36(3):140–7.
Kumar M, Misra A, Mishra AK, Mishra P, Pathak K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J Drug Target. 2008;16(10):806–14.
Prajapati ST, Pathak SP, Thakkar JH, Patel CH. Nanoemulsion based intranasal delivery of risperidone for nose to brain targeting. Bull Pharmaceut Res. 2015;5(1):6–13.
Nazar H, Fatouros DG, van der Merwe SM, Bouropoulos N, Avgouropoulos G, Tsibouklis, et al. Thermosensitive hydrogels for nasal drug delivery: the formulation and characterisation of systems based on N-trimethyl chitosan chloride. Eur J Pharm Biopharm. 2011;77(2):225–32.
Jalalvand E, Robertson B, Tostivint H, Löw P, Wallén P, Grillner S. Cerebrospinal fluid-contacting neurons sense Ph changes and motion in the hypothalamus. J Neurosci. 2018;38(35):7713–24.
Nigam K, Kaur A, Manda K, Tyagi A, Gabrani R, Dang S. Baclofen-loaded PLGA nanoparticles for neuropathic pain management: in-vitro and in-vivo evaluation. Rejuvenation Res. 2018;22(3):235–45. https://doi.org/10.1089/rej.2018.2119.
Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014;21(2):148–54.
Patil NL, Mahajan HS. Quercetin loaded nanostructured lipid carriers for nose to brain delivery: in vitro and in vivo studies. Am J Adv Drug Delivery. 2018;6(1):0091–00920.
Saroha K, Singh S, Aggarwal A, Nanda S. Transdermal gels – an alternative vehicle for drug delivery. Int J Pharmaceut Chem Biol Sci 2013;3(3):495–503.
Shahavi MS, Hosseini M, Jahanshahi M, Meyer RL, Darzi GN. Clove oil nanoemulsion as an effective antibacterial agent: Taguchi optimization method. J Desalin Water Treat. 2015;57(39):1–12.
Singh S, Bothara SB. Development of oral mucoadhesive tablets of losartan potassium using natural gum from Manilkara Zapota seeds. Int J Pharmaceut Sci Nanotechnol. 2013;6(4):2245–54.
Khan MW, Ahsan MJ, Gupta SK. Development and In vitro characterization of mucoadhesive nanoemulgel (MNEG) for enhanced delivery of carbamazepine”. Int J Adv Pharm Med Bioallied Sci. 2017;2017(2017):1–9.
Gupta M, Vyas SP. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem Phys Lipid. 2012;165:454–61.
Householder KT, Dharmaraj S, Sandberg DI, Wechsler-Reya RJ, Sirianni RW. Fate of nanoparticles in the central nervous system after intrathecal injection in healthy mice. Sci Rep. 2019;9(1):12587.
Alam T, Pandit J, Vohora D, Aqil M, Ali A, Sultana Y. Optimization of nanostructured lipid carriers of lamotrigine for brain delivery: in vitro characterization and in vivo efficacy in epilepsy. Expert Opin Drug Deliv. 2015;12(2):181–94.
Salem HF, Kharshoum RM, Abou-Taleb HA, Naguib DM. Nanosized nasal emulgel of resveratrol: preparation, optimization, in vitro evaluation and in vivo pharmacokinetic study. Drug Dev Ind Pharm. 2019;45(10):1624–34.
Clementino A, Batger M, Garrastazu G, Pozzoli M, Del Favero E, Rondelli V, et al. The nasal delivery of nanoencapsulated statins-an approach for brain delivery. Int J Nanomed. 2016;11:6575–90.
Rencber S, Karwana SY, Yilmaz FF, Erac B, Nenni M, Ozbal S, et al. Development, characterization, and in vivo assessment of mucoadhesive nanoparticles containing fluconazole for the local treatment of oral candidiasis. Int J Nanomed. 2020;10(11):2641–53.
Singh S, Ramaiah M, Devgan M, Sarkar BK. Phytochemical and comparative antioxidant evaluation by DPPH and reducing power assay of Hybanthus Enneaspermus. Acta Sci Pharmaceut Sci. 2017;1(1):01–4.
Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia Terapotensis. J Nat Prod. 2001;64:892–5.
Qiang L, Yang Y, Ma YJ, Chen FH, Zhang LB, Liu W, et al. Isolation and characterization of cancer stem like cells in human glioblastoma cell lines. Cancer Lett. 2009;279(1):13–21. https://doi.org/10.1016/j.canlet.2009.01.016.
Sharma A, Gupta S, Sarethy IP, Dang S, Gabrani R. Green tea extract: possible mechanism and antibacterial activity on skin pathogens. Food Chem. 2012;135(2):672–5.
Chan JM, Zhang L, Yuet KP, Liao G, Rhee JW, Langer R, et al. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30(8):1627–34.
Singh S, Singh R, Kushwah AS, Gupta G. Neuroprotective role of antioxidant and pyranocarboxylic acid derivative against AlCl3 induced Alzheimer’s disease in rats. J Coast Life Med . 2014;2(7):571–8.
Ogasawara Y, Sakamoto T, Ishii K, Takahashi H, Tanabe S. Effects of the administration routes and chemical forms of aluminium on aluminium accumulation in rat brain. Biol Trace Elem Res. 2002;86(3):269–78.
Dews PB. The measurement of the influence of drugs on voluntary activity in mice. Brit J Pharmacy Chemotherap. 1953;8:46–8.
Prabhakar S, Saraf MK, Banik A, Anand A. Bacopa monniera selectively attenuates suppressed Superoxide dismutase activity in diazepam induced amnesic mice. Ann Neurosci. 2011;18(1):8–13.
Reddy DS, Kulkarni SK. Possible role of nitric oxide in the nootropic and antiamnesic effects of neurosteroids on aging and dizocilipine induced learning impairment. Brain Res. 1998;799:215–29.
Ataie A, Sabetkassaei M, Haghparast A, Moghaddam A, Alaee R, Sn M. Curcumin exerts neuroprotective effects against homocysteine intracerebroventricular injection-induced cognitive impairment and oxidative stress in rat brain. J Med Food. 2010;13(4):821–6.
Cook L, Weidley E. Behavioral effects of some psychopharmacological agents. Ann N Y AcadSci. 1957;66(3):740–52.
Soman I, Mengi SA, Kasture SB. Effect of leaves of Buteafrondosa on stress, anxiety, and cognition in rats. Pharmacol Biochem Behav. 2004;79(1):11–6.
Toledo-Morrell L, Morrell F, Fleming S. Age-dependent deficits in spatial memory are related to impaired hippocampal kindling. Behav Neurosci. 1984;98:902–7.
Ikegami S. Behavioral impairment in radial-arm maze learning and acetylcholine content of the hippocampus and cerebral cortex in aged mice. Behav Brain Res. 1994;65:103–11.
Dhingra D, Parle M, Kulkarni SK. Memory enhancing activity of Glycyrrhiza glabra in mice. J Ethnopharmacol. 2004;91(2–3):361–5.
Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plus-maze for the evaluation of memory in mice: effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology. 1990;101(1):27–33.
Parle M, Dhingra D. Ascorbic acid: a promising memory- enhancer in mice. J Pharmacol Sci. 2003;93(2):129–35.
Rouini MR, Asadipour A, Ardakani YH, Aghdasi F. Liquid chromatography method for determination of mefenamic acid in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;800(1–2):189–92. https://doi.org/10.1016/j.jchromb.2003.09.063.
Uddin ABM, Jm Mohamad, Al-Aama M, Amiruddin N. High performance liquid chromatographic determination of mefenamic acid in human plasma using UV vis detector. Int J Pharm Sci. 2014;6(11):167–70.
Shah B, Khunt D, Misra M, Padh N. Non-invasive intranasal delivery of quetiapine fumarate loaded microemulsion for brain targeting: formulation, physicochemical and pharmacokinetic consideration. Eur J Pharm Sci. 2016;91:196–207.
Etman MA, Farid RM, Nada AH, Ebian AAR. In vitro/in vivo correlation of fast release mephenamic acid microspheres in humans. Med Princ Pract. 2012;21:223–7.
Abdou EM, Kandil S, Miniawy H. Brain targeting efficiency of antimigraine drug loaded mucoadhesive intranasal nanoemulsion. Int J Pharm. 2017;529:667–77.
Girotra P, Singh SK, Kumar G. Development of zolmitriptan loaded PLGA/ poloxamer nanoparticles for migraine using quality by design approach. International Journal of biological Macromolecues. 2016;85:92–101.
Haque S, Md S, Sahni JK, Ali J, Baboota S. Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J Psychiatr Res. 2014;48(1):1–12.
Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667–70.
Jollow DI, Mitchell JR, Zampaglione N, Gillette JR. Bromobenze induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–69.
Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95.
Claiborne A. Catalase activity. In: Greenwald RA, editor. Handbook of Methods for Oxygen Radical Research. USA: CRC Press, Boca Raton, FL; 1985. p. 283–4.
Ellman GL, Courtney DK, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95.
Kumar R, Kumar R, Sharma N, Khurana N. Ameliorative effect of myrcene in mouse model of Alzheimer’s disease. Eur J Pharmacol. 2021;911.
Humason GL. Freeman WH. San Francisco: Animal tissue technique; 1962. p. 3–126.
Das S, Samanta A, Bose A. Design, development and evaluation of fluconazole topical gel. Asian J Pharm Clin Res. 2015;8(2):132–5.
Chandra R, Sanghi A, Kumar D, Singh V. A simultaneous method validation for the estimation of mefenamic acid from marketed tablets by reversed phase high performance liquid chromatography (RP-HPLC) and UV-VIS spectrophotometer. Pharm Lett. 2014;6(4):138–42.
Rodrigues IA, de Ramos SA, Falcão DQ, Ferreira JLP, Basso SL, de Silva A, et al. Development of nanoemulsions to enhance the antileishmanial activity of Copaifera nti-i oleoresins. Biomed Res Int. 2018;2018:1–9.
Sánchez-López E, Guerra M, Dias-Ferreira J, Lopez-Machado A, Ettcheto M, Cano A, et al. Current applications of nanoemulsions in cancer therapeutics. Nanomaterials. 2019;9:821.
Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231(25):232–5.
Chaiamnuay S, Allison JJ, Curtis JR. Risks versus benefits of cyclooxygenase-2-selective nonsteroidal nti-inflammatory drugs. Am J Health Syst Pharm. 2006;63(19):1837–51.
Ghosh MN. Guide to drug doses in laboratory animals fundamentals of experimental Pharmacology. 3rd ed. Calcutta: Hilton and Company; 2005. p. 191–201.
Dhuyvetter D, Tekle F, Nazarov M, Vreeken RJ, Borghys H, Rombouts F, et al. Direct nose to brain delivery of small molecules: critical analysis of data from a standardized in vivo screening model in rats. Drug Delivery. 2020;27(1):1597–607.
Chhonker YS, Chandasana H, Kumar A, Kumar D, Laxman TS, Mishra SK, et al. Pharmacokinetics, tissue distribution and plasma protein binding studies of rohitukine: a potent anti-hyperlipidemic agent. Drug Res (Stuttg). 2015;65(7):380–7.
Dhumal BR, Bhusari KP, Tajne MR, Ghante MH, Jain NS. Stability indicating method for the determination of mefenamic acid in pharmaceutical formulations by HPLC. Journal of Applied Pharmaceutical Science. 2014;4(12):60–4.
Di Stefano A, Iannitelli A, Laserra S, Sozio P. Drug delivery strategies for Alzheimer’s disease treatment. Expert Opin Drug Deliv. 2011;8:581–603.
Mefenamic acid, drug description. Available at: https://www.rxlist.com/mefenamic-acid-drug.htm#description (accessed January 7, 2023).
McGeer PL, McGeer EG. Anti-inflammatory drugs in the fight against Alzheimer’s disease. Ann NY Acad Sci. 1996;1997:213–20.
Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A. Nanoemulsion based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014;21:148–54.
Attwood D, Mallon C, Ktistis G, Taylor CJ. A study on factors influencing the droplet size in nti-infl oil-in-water microemulsions. Int J Pharm. 1992;88:417–22.
Pandey YR, Kumar S, Gupta BK, Ali J, Baboota S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: formulation, behavioural and biochemical estimation. Nanotechnology. 2015;27.
Pathak R, Prasad Dash R, Misra M, Nivsarkar M. Role of mucoadhesive polymers in enhancing delivery of nimodipine microemulsion to brain via intranasal route. Acta Pharm Sin B. 2014;4(2):151–60.
Yokel RA. Blood brain barrier flux of aluminium, managanese, iron and other metals suspected to contribute to metal-induced neurodegeneration. J Alzhiemers Dis. 2006;10:223–53.
Ravi SM, Prabhu BM, Raju TR, Bindu PN. Long-term effects of postnatal aluminium exposure on acetylcholinestrase activity and biogenic amine neurotransmitters in rat brain. Indian J Physiol Pharmacol. 2000;44:473–8.
Pal A, Choudhary M, Joshi DK, Tripathi S, Modi DR. Alteration in thyroid hormones and vitamins as early markers of aluminium induced neurodegeneration in rats. Int J Res Pharm Sci. 2012;2(2):137–50.
Wagner JM, Sichler ME, Schleicher EM, Franke TN, Irwin C, Löw MJ, et al. Analysis of motor function in the Tg4-42 mouse model of Alzheimer’s disease. 2019;13:1-13.
Wirths O, Bayer TA. Motor impairment in Alzheimer’s disease and transgenic Alzheimer’s disease mouse models. 2008;7:1–5.
Reddy DS. Assesment of nootropic and amnestic activity of centrally acting agents. Ind J Pharmacol. 1997;29(4):208–21.
Rao SB, Chetana M, Devi PU. Centella asiat-ica treatment during postnatal period enhances learning and memory in mice. Physiol Behav. 2005;86:449–57.
Al-Amin MM, Reza HM, Saadi HM, Mahmud W, Ibrahim AA, Alam MM, et al. Astaxanthin amelio-rates nti-infl chloride-induced spatial memory impairment and neuronal oxidative stress in mice. Euro J Pharmacol. 2016;777:60–9.
Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60.
Berthon G. Aluminum speciation in relation to aluminium bioavailability, metabolism and toxicity. Coord Chem Rev. 2002;228(2):319–41.
Ohtawa M, Seko M, Takayama F. Effect of aluminium ingestion on lipid peroxidation in rats. Chem Pharm Bull. 1983;31:1415–8.
Singh A, Ujjwal RR, Naqvi S, Verma RK, Tiwari S, Kesharwani P, et al. Formulation development of tocopherol polyethylene glycol nanoengineered polyamidoamine dendrimer for neuroprotection and treatment of Alzheimer disease. J Drug Target. 2022;18:1–15.
Fransen M, Nordgern M, Wang B, Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim Biophys Acta. 2012;1822(9):1363–73.
Sies H. Role of metabolic H2O2 generation: redox signalling and oxidative stress. J Biol Chem. 2014;289(13):8735–41.
Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;6:192–208.
Ballard CG, Greig NH, Guillozet-Bongaarts AL, Enz A, Darvesh S. Cholinesterases: roles in the brain during health and disease. Curr Alzheimer Res. 2005;2:307–18.
Mattson MP. Pathways towards and away from Alzheimer’s disease. Nat. 2004;430:631–9.
Vassar R. BACE1: the β-secretase enzyme in Alzheimer’s disease. J Mol Neurosci. 2004;23:105–14.
Dragicevic N, Smith A, Lin X, Yuan F, Copes N, Delic V, et al. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer’s amyloid-induced mitochondrial dysfunction. J Alzheimers Dis. 2011; 26:507e521.
Sarkar P, Zaja I, Bienengraeber M, Rarick KR, Terashvili M, Canfield S, et al. Epoxyeicosatrienoic acids pretreatment improves amyloidbeta induced mitochondrial dysfunction in cultured rat hippocampal astrocytes. Am J Physiol Heart Circ Physiol. 2014;306:H475Eh484.
Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26:7212–21.
Li F, Gong QH, Wu Q, Lu YF, Shi JS. Icariin isolated from Epimedium brevicornum Maxim attenuates learning and memory deficits induced by D-galactose in rats. Pharmacol Biochem Behav. 2010;96:301–3.
Acknowledgements
The authors acknowledge the support and facilities provided by Khalsa College of Pharmacy, Amritsar, in carrying out the present research work.
Author information
Authors and Affiliations
Contributions
Jasjeet Kaur Narang: conceptualisation, methodology, supervision. Anmol Dogra: investigation, data curation, writing—original draft preparation. Ramandeep Singh Narang: data curation, formal analysis. Tajpreet Kaur: supervision, formal analysis.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dogra, A., Narang, R.S., Kaur, T. et al. Mefenamic Acid Loaded and TPGS Stabilized Mucoadhesive Nanoemulsion for the Treatment of Alzheimer’s Disease: Development, Optimization, and Brain-Targeted Delivery via Olfactory Pathway. AAPS PharmSciTech 25, 16 (2024). https://doi.org/10.1208/s12249-023-02727-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02727-0