Abstract
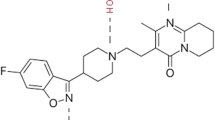
By selecting L-arginine as the hydrogen bond acceptor (HBA) and 2-hydroxypropyl-β-cyclodextrin (2HPβCD) as the hydrogen bond donor (HBD), deep eutectic solvents (DESs) with various water content were prepared at the 4:1 mass ratio of L-arginine to 2HPβCD with 40 to 60% of water, and were studied for its application in transdermal drug delivery system (TDDS). The hydrogen bond networks and internal chemistry structures of the DESs were measured by attenuated total reflection Fourier transform infrared (ATR-FTIR) and 1H-nuclear magnetic resonance spectroscopy (1H-NMR), which demonstrated the successful synthesis of DESs. The viscosity of DES was decreased from 10,324.9 to 3219.6 mPa s, while glass transition temperature (Tg) of the DESs was increased from − 60.8 to − 51.4 °C, as the added water was increased from 45 to 60%. The solubility of ibuprofen, norfloxacin, and nateglinide in DES with 45% of water were increased by 79.3, 44.1, and 3.2 times higher than that in water, respectively. The vitro study of transdermal absorption of lidocaine in DESs showed that the cumulative amounts of lidocaine reached 252.4 µg/cm2, 226.1 µg/cm2, and 286.1 µg/cm2 at 8 h for DESs with 45%, 50%, and 60% of water, respectively. The permeation mechanism of DES with lower content of water (45%) was mainly by changing the fluidization of lipids, while changing the secondary structure of keratin in stratum corneum (SC) at higher water content (50% and 60%). These nonirritant and viscous fluid like DESs with good drug solubility and permeation enhancing effects have broad application prospect in the field of drug solubilization and transdermal drug delivery system.
Graphical Abstract













Similar content being viewed by others
Data Availability
Data available on request from the authors.
References
Ramadon D, McCrudden MT, Courtenay AJ, Donnelly RF. Enhancement strategies for transdermal drug delivery systems: current trends and applications. Drug Deliv Transl Re. 2022;12(4):758–91.
Zaid Alkilani A, McCrudden MT, Donnelly RF. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7(4):438–70.
Qindeel M, Ullah MH, Ahmed N. Recent trends, challenges and future outlook of transdermal drug delivery systems for rheumatoid arthritis therapy. J Control Release. 2020;327:595–615.
Roohnikan M, Laszlo E, Babity S, Brambilla D. A snapshot of transdermal and topical drug delivery research in Canada. Pharmaceutics. 2019;11(6):256.
Peña-Juárez M, Guadarrama-Escobar OR, Escobar-Chávez JJ. Transdermal delivery systems for biomolecules. J Pharm Innov. 2022;17(2):319–32.
Leppert W, Malec-Milewska M, Zajaczkowska R, Wordliczek J. Transdermal and topical drug administration in the treatment of pain. Molecules. 2018;23(3):681.
Jiang T, Xu G, Chen G, Zheng Y, He B, Gu Z. Progress in transdermal drug delivery systems for cancer therapy. Nano Res. 2020;13(7):1810–24.
Kováčik A, Kopečná M, Vávrová K. Permeation enhancers in transdermal drug delivery: benefits and limitations. Expert Opin Drug Del. 2020;17(2):145–55.
Dragicevic N, Maibach H. Combined use of nanocarriers and physical methods for percutaneous penetration enhancement. Adv Drug Deliv Rev. 2018;127:58–84.
Roberts M, Mohammed Y, Pastore M, Namjoshi S, Yousef S, Alinaghi A, et al. Topical and cutaneous delivery using nanosystems. J Control Release. 2017;247:86–105.
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem Comm. 2003;1:70–1.
Zainal-Abidin MH, Hayyan M, Ngoh GC, Wong WF, Looi CY. Emerging frontiers of deep eutectic solvents in drug discovery and drug delivery systems. J Control Release. 2019;316:168–95.
Smith EL, Abbott AP, Ryder KS. Deep eutectic solvents (DESs) and their applications. Chem Rev. 2014;114(21):11060–82.
Hattori T, Tagawa H, Inai M, Kan T, Kimura S-I, Itai S, et al. Transdermal delivery of nobiletin using ionic liquids. Sci Rep. 2019;9(1):1–11.
Daadoue S, Al-Remawi M, Al-Mawla L, Idkaidek N, Khalid RM, Al-Akayleh F. Deep eutectic liquid as transdermal delivery vehicle of Risperidone. J Mol Liq. 2022;345:117347.
Wang C, Yan T, Yan T, Wang Z. Fabrication of hesperetin/hydroxypropyl-β-cyclodextrin complex nanoparticles for enhancement of bioactivity using supercritical antisolvent technology. J Mol Struct. 2023;1279:134947.
Donthi MR, Munnangi SR, Krishna KV, Marathe SA, Saha RN, Singhvi G, Dubey SK. Formulating ternary inclusion complex of sorafenib tosylate using β-cyclodextrin and hydrophilic polymers: physicochemical characterization and in vitro assessment. AAPS PharmSciTech. 2022;23(7):254.
Mohandoss S, Velu KS, Stalin T, Ahmad N, Alomar SY, Lee YR. Tenofovir antiviral drug solubility enhancement with β-cyclodextrin inclusion complex and in silico study of potential inhibitor against SARS-CoV-2 main protease (Mpro). J Mol Liq. 2023;377:121544.
Cai C, Yu W, Wang C, Liu L, Li F, Tan Z. Green extraction of cannabidiol from industrial hemp (Cannabis sativa L.) using deep eutectic solvents coupled with further enrichment and recovery by macroporous resin. J Mol Liq. 2019;287:110957.
Lv J, Ou X, Fang Y, Wu M, Zheng F, Shang L, et al. The study of deep eutectic solvent based on choline chloride and l-(+)-tartaric acid diethyl ester for transdermal delivery system. AAPS PharmSciTech. 2022;23(7):1–10.
Shang L, Cun D, Xi H, Fang L. An explanation for the difference in the percutaneous penetration behavior of tamsulosin induced by two different O-acylmenthol derivatives. AAPS PharmSciTech. 2014;15(4):803–9.
Kobayashi I, Hosaka K, Ueno T, Maruo H, Kamiyama M, Konno C, et al. Relationship between the amount of propranolol permeating through the stratum corneum of guinea pig skin after application of propranolol adhesive patches and skin irritation. Biol Pharm Bull. 1996;19(6):839–44.
Roda A, Santos F, Chua YZ, Kumar A, Do HT, Paiva A, et al. Unravelling the nature of citric acid: L-arginine: water mixtures: the bifunctional role of water. Chem Chem Phys. 2021;23(2):1706–17.
Zhu G, Xiao Z, Zhu G. Fabrication and characterization of ethyl acetate–hydroxypropyl-β-cyclodextrin inclusion complex. J Food Sci. 2021;86(8):3589–97.
Sun C, Cao J, Wang Y, Chen J, Huang L, Zhang H, et al. Ultrasound-mediated molecular self-assemble of thymol with 2-hydroxypropyl-β-cyclodextrin for fruit preservation. Food Chem. 2021;363:130327.
Tanner EE, Curreri AM, Balkaran JP, Selig-Wober NC, Yang AB, Kendig C, et al. Design principles of ionic liquids for transdermal drug delivery. Adv Mater. 2019;31(27):1901103.
Xu LH, Wu D, Zhong M, Wang GB, Chen XY, Zhang ZJ. The construction of a new deep eutectic solvents system based on choline chloride and butanediol: the influence of the hydroxyl position of butanediol on the structure of deep eutectic solvent and supercapacitor performance. J Power Sources. 2021;490:229365.
Zinov’eva I, Fedorov AY, Milevskii N, Zakhodyaeva YA, Voshkin A. A deep eutectic solvent based on choline chloride and sulfosalicylic acid: properties and applications. Theor Found Chem Eng. 2021;55(3):371–9.
Al-Sadat W, Nasser M, Chang F, Nasr-El-Din H, Hussein I. Rheology of a viscoelastic zwitterionic surfactant used in acid stimulation: effects of surfactant and electrolyte concentration. J Pet Sci Eng. 2014;124:341–9.
Altamash T, Nasser MS, Elhamarnah Y, Magzoub M, Ullah R, Qiblawey H, et al. Gas solubility and rheological behavior study of betaine and alanine based natural deep eutectic solvents (NADES). J Mol Liq. 2018;256:286–95.
Rahman MS, Roy R, Montoya C, Halim MA, Raynie DE. Acidic and basic amino acid-based novel deep eutectic solvents and their role in depolymerization of lignin. J Mol Liq. 2022;362:119751.
Dai Y, van Spronsen J, Witkamp G-J, Verpoorte R, Choi YH. Natural deep eutectic solvents as new potential media for green technology. Anal Chim Acta. 2013;766:61–8.
Mohandoss S, Edison TNJI, Atchudan R, Palanisamy S, Prabhu NM, Napoleon AA, et al. Ultrasonic-assisted efficient synthesis of inclusion complexes of salsalate drug and β-cyclodextrin derivatives for potent biomedical applications. J Mol Liq. 2020;319:114358.
Preskar M, Vrbanec T, Vrečer F, Šket P, Plavec J, Gašperlin M. Solubilization of ibuprofen for freeze dried parenteral dosage forms. Acta Pharm. 2019;69(1):17–32.
Deaconu M, Pintilie L, Vasile E, Mitran R-A, Pircalabioru GG, Matei C, et al. Norfloxacin delivery systems based on MCM-type silica carriers designed for the treatment of severe infections. Mater Chem Phys. 2019;238:121886.
Boddu P, Cherakapu VL, Ponukumati U. Application of solid dispersion technique in solubility and dissolution rate enhancement of nateglinide. Asian J Pharm Clin Res. 2017;10(11):231–8.
Domańska U, Pelczarska A, Pobudkowska A. Effect of 2-hydroxypropyl-β-cyclodextrin on solubility of sparingly soluble drug derivatives of anthranilic acid. Int J Mol Sci. 2011;12(4):2383–94.
Kaushik D, Michniak-Kohn B. Percutaneous penetration modifiers and formulation effects: thermal and spectral analyses. AAPS PharmSciTech. 2010;11(3):1068–83.
Salimi A, Hedayatipour N, Moghimipour E. The effect of various vehicles on the naproxen permeability through rat skin: a mechanistic study by DSC and FT-IR techniques. Adv Pharm Bull. 2016;6(1):9.
Kosuge M, Takeuchi T, Nakase I, Jones AT, Futaki S. Cellular internalization and distribution of arginine-rich peptides as a function of extracellular peptide concentration, serum, and plasma membrane associated proteoglycans. Bioconjug Chem. 2008;19(3):656–64.
Ahmadi R, Hemmateenejad B, Safavi A, Shojaeifard Z, Mohabbati M, Firuzi O. Assessment of cytotoxicity of choline chloride-based natural deep eutectic solvents against human HEK-293 cells: a QSAR analysis. Chemosphere. 2018;209:831–8.
Funding
The authors received financial supports from the Key-Area Research and Development Program of Guangdong Province (grant number 2020B1111590001); “Dengfeng Plan” High-level Hospital Construction Opening Project of Foshan Hospital of Traditional Chinese Medicine (grant number 202000192); the Youth Innovation Fund of the Jihua Laboratory, China (grant number X201251XL200); Young Top Talents of Liaoning Xingliao Talents Program (grant number XLYC2007184); and Young and Middle-aged Scientific and Technological Innovation Talents Plan in Shenyang (grant number RC200367).
Author information
Authors and Affiliations
Contributions
Jianhua Lv: conceptualization, methodology, investigation, formal analysis, data curation, writing. Pan Wu: methodology, investigation. Yaru Fang: methodology, investigation. Wenchang Zhang: methodology, investigation. Dongwen Liu: methodology, investigation. Mi Wu: formal analysis, soft ware. Lei Shang: resources, date collection, date analysis, writing—review. Huaiguo Li: resources, writing—review. Yan Zhao: resources, date analysis, writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, J., Wu, P., Fang, Y. et al. Deep Eutectic Solvents Based on L-Arginine and 2-Hydroxypropyl-β-Cyclodextrin for Drug Carrier and Penetration Enhancement. AAPS PharmSciTech 24, 187 (2023). https://doi.org/10.1208/s12249-023-02638-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02638-0