Abstract
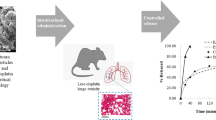
Hydroxychloroquine (HCQ) was repurposed for COVID-19 treatment. Subtherapeutic HCQ lung levels and cardiac toxicity of oral HCQ were overcome by intratracheal (IT) administration of lower HCQ doses. The crosslinker-free supercritical fluid technology (SFT) produces aerogels and impregnates them with drugs in their amorphous form with efficient controlled release. Mechanistic physiologically based pharmacokinetic (PBPK) modeling can predict the lung’s epithelial lining fluid (ELF) drug levels. This study aimed to develop a novel HCQ SFT formulation for IT administration to achieve maximal ELF levels and minimal cardiac toxicity. HCQ SFT formulation was prepared and evaluated for physicochemical, in vitro release, pharmacokinetics, and cardiac toxicity. Finally, the rat HCQ ELF concentrations were predicted using PBPK modeling. HCQ was amorphous after loading into the chitosan-alginate nanoporous microparticles (22.7±7.6 μm). The formulation showed a zero-order release, with only 40% released over 30 min compared to 94% for raw HCQ. The formulation had a tapped density of 0.28 g/cm3 and a loading efficiency of 35.3±1.3%. The IT administration of SFT HCQ at 1 mg/kg resulted in 23.7-fold higher bioavailability, fourfold longer MRT, and eightfold faster absorption but lower CK-MB and LDH levels than oral raw HCQ at 4 mg/kg. The PBPK model predicted 6 h of therapeutic ELF levels for IT SFT HCQ and a 100-fold higher ELF-to-heart concentration ratio than oral HCQ. Our findings support the feasibility of lung-targeted and more effective SFT HCQ IT administration for COVID-19 compared to oral HCQ with less cardiac toxicity.

Graphical abstract





Similar content being viewed by others
References
da Silva AEA, de Abreu PMB, Geraldes DC, de Oliveira NL. Hydroxychloroquine: pharmacological, physicochemical aspects and activity enhancement through experimental formulations. J Drug Deliv Sci Technol. 2021;63:102512. https://doi.org/10.1016/j.jddst.2021.102512.
Concardia. Plaquenil New Drug Application. 2007. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=009768. Accessed 23 Aug 2022.
Perinel S, Launay M, Botelho-Nevers É, Diconne É, Louf-Durier A, Lachand R, et al. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. Clin Infect Dis. 2020;71:2227–9. https://doi.org/10.1093/cid/ciaa394.
Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–7. https://doi.org/10.1016/j.ijid.2020.01.050.
World Health Orgnization. WHO coronavirus (COVID-19) dashboard. 2023. https://covid19.who.int/. Accessed 16 June 2023.
Kingsbury SR, Tharmanathan P, Adamson J, Arden NK, Birrell F, Cockayne S, Dickson J, Doherty M, Dziedzic KS, Grainger A, Hewitt CE, O'Neill TW, Scott DL, Vincent TL, Wakefield RJ, Watt FE, Torgerson DJ, Conaghan PG. Hydroxychloroquine effectiveness in reducing symptoms of hand osteoarthritis (HERO): study protocol for a randomized controlled trial. Trials Journal. 2013;14:12. https://doi.org/10.1186/1745-6215-14-64.
Browning DJ. Pharmacology of chloroquine and hydroxychloroquine. Hydroxychloroquine and chloroquine retinopathy. New York, NY: Springer; 2014. p. 35–63.
Tett S, Cutler D, Day R, Brown K. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1988;26:303–13. https://doi.org/10.1111/j.1365-2125.1988.tb05281.x.
Ferreira A, Junior RTLM, Rosa TA, Rolim LA, Rolim-Neto PJ. Clinical, pharmacokinetic and technological aspects of the hydroxychloroquine sulfate. IOSR J Pharm. 2014;4:53–64.
Beau B, Furst DE. Hydroxychloroquine. In: Day RO, Fürst DE, Riel PLCM, Bresnihan B, editors. Antirheumatic therapy: actions and outcomes. 1st ed. Progress in Inflammation Research. Basel, switzerland: Birkhäuser Basel; 2005. p. 81-92.
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71:732–9. https://doi.org/10.1093/cid/ciaa237.
McLachlan A, Cutler D, Tett S. Plasma protein binding of the enantiomers of hydroxychloroquine and metabolites. Eur J Clin Pharmacol. 1993;44:481–4. https://doi.org/10.1007/BF00315548.
Collins KP, Jackson KM, Gustafson DL. Hydroxychloroquine: a physiologically-based pharmacokinetic model in the context of cancer-related autophagy modulation. J Pharmacol Exp Ther. 2018;365:447–59. https://doi.org/10.1124/jpet.117.245639.
Rendic S, Guengerich FP. Metabolism and interactions of chloroquine and hydroxychloroquine with human cytochrome P450 enzymes and drug transporters. Curr Drug Metab. 2020;21:1127–35. https://doi.org/10.2174/1389200221999201208211537.
Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127–0. https://doi.org/10.1128/JVI.00127-20.
Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–60. https://doi.org/10.1007/s11427-020-1637-5.
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11:875–9. https://doi.org/10.1038/nm1267.
Arnold SLM, Buckner F. Hydroxychloroquine for treatment of SARS-CoV-2 infection? Improving our confidence in a model-based approach to dose selection. Clin Transl Sci. 2020;13:642–5. https://doi.org/10.1111/cts.12797.
Kiem S, Schentag JJ. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother. 2008;52:24–36. https://doi.org/10.1128/AAC.00133-06.
Franciosi L, Govorukhina N, Ten Hacken N, Postma D, Bischoff R. Proteomics of epithelial lining fluid obtained by bronchoscopic microprobe sampling. Methods Mol Biol. 2011;790:17–28. https://doi.org/10.1007/978-1-61779-319-6_2.
Wilson KC, Chotirmall SH, Bai C, Rello J. COVID-19: interim guidance on management pending empirical evidence. From an American Thoracic Society-led International Task Force. 2020:12.
Yoon YH, Cho KS, Hwang JJ, Lee S-J, Choi JA, Koh J-Y. Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Invest Ophthalmol Vis Sci. 2010;51:6030–7. https://doi.org/10.1167/iovs.10-5278.
Fassihi SC, Nabar NR, Fassihi R. Novel approach for low-dose pulmonary delivery of hydroxychloroquine in COVID-19. COVID-19. Research. 2020;177:4997–8. https://doi.org/10.1111/bph.15167.
Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–7. https://doi.org/10.1016/j.healun.2020.03.012.
Hawari F, Dodin Y, Tayyem R, Najjar S, Kakish H, Fara MA, et al. Safety, tolerability, and pharmacokinetics of nebulized hydroxychloroquine: a pilot study in healthy volunteers. J Aerosol Med Pulm Drug Deliv. 2023;36:76–81. https://doi.org/10.1089/jamp.2022.0062.
Chen G, Wu DI, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–9. https://doi.org/10.1172/JCI137244.
Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020;55:105982. https://doi.org/10.1016/j.ijantimicag.2020.105982.
Klimke A, Hefner G, Will B, Voss U. Hydroxychloroquine as an aerosol might markedly reduce and even prevent severe clinical symptoms after SARS-CoV-2 infection. Med Hypotheses. 2020;142:109783. https://doi.org/10.1016/j.mehy.2020.109783.
Nasir Idkaidek FH, Dodin Y, Obeidat N. Development of a physiologically-based pharmacokinetic (PBPK) model of nebulized hydroxychloroquine for pulmonary delivery to COVID-19 patients. Drug Res. 2020;71:250–6. https://doi.org/10.1055/a-1325-0248.
Maharaj AR, Wu H, Hornik CP, Balevic SJ, Hornik CD, Smith PB, et al. Simulated assessment of pharmacokinetically guided dosing for investigational treatments of pediatric patients with coronavirus disease 2019. JAMA Pediatr. 2020;174:e202422-e. https://doi.org/10.1001/jamapediatrics.2020.2422.
Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers Y-M. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41:919–31. https://doi.org/10.1007/s40264-018-0689-4.
Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1036–41. https://doi.org/10.1001/jamacardio.2020.1834.
Ramireddy A, Chugh H, Reinier K, Ebinger J, Park E, Thompson M, et al. Experience with hydroxychloroquine and azithromycin in the coronavirus disease 2019 pandemic: implications for QT interval monitoring. J Am Heart Assoc. 2020;9:e017144. https://doi.org/10.1161/JAHA.120.017144.
Kavanagh O, Healy AM, Dayton F, Robinson S, O’Reilly NJ, Mahoney B, et al. Inhaled hydroxychloroquine to improve efficacy and reduce harm in the treatment of COVID-19. Med Hypotheses. 2020;143:110110. https://doi.org/10.1016/j.mehy.2020.110110.
Albariqi AH, Chang RYK, Tai W, Ke W-R, Chow MY, Tang P, et al. Inhalable hydroxychloroquine powders for potential treatment of COVID-19. J Aerosol Med Pulm Drug Deliv. 2021;34:20–31. https://doi.org/10.1089/jamp.2020.1648.
Ali AS, Alrashedi MG, Ahmed OAA, Ibrahim IM. Pulmonary delivery of hydroxychloroquine nanostructured lipid carrier as a potential treatment of COVID-19. Polymers. 2022;14:2616. https://doi.org/10.3390/polym14132616.
Deshpande PB, Kumar GA, Kumar AR, Shavi GV, Karthik A, Reddy MS, et al. Supercritical fluid technology: concepts and pharmaceutical applications. PDA J Pharm Sci Technol. 2011;65:333–44. https://doi.org/10.5731/pdajpst.2011.00717.
Badens E, Majerik V, Horváth G, Szokonya L, Bosc N, Teillaud E, et al. Comparison of solid dispersions produced by supercritical antisolvent and spray-freezing technologies. Int J Pharm. 2009;377:25–34. https://doi.org/10.1016/j.ijpharm.2009.04.047.
Schmitt WJ, Reid RC. The use of entrainers in modifying the solubility of phenanthrene and benzoic acid in supercritical carbon dioxide and ethane. Fluid Phase Equilib. 1986;32:77–99. https://doi.org/10.1016/0378-3812(86)87007-8.
Kalani M, Yunus R. Application of supercritical antisolvent method in drug encapsulation: a review. Int J Nanomedicine. 2011;6:9–42. https://doi.org/10.2147/IJN.S19021.
Davies OR, Lewis AL, Whitaker MJ, Tai H, Shakesheff KM, Howdle SM. Applications of supercritical CO 2 in the fabrication of polymer systems for drug delivery and tissue engineering. Adv Drug Deliv Rev. 2008;60:373–87. https://doi.org/10.1016/j.addr.2006.12.001.
Braga ME, Pato MTV, Silva HSC, Ferreira EI, Gil MH, Duarte CM, et al. Supercritical solvent impregnation of ophthalmic drugs on chitosan derivatives. J Supercrit Fluids. 2008;44:245–57. https://doi.org/10.1016/j.supflu.2007.10.002.
Tang Q, Wang T. Preparation of silica aerogel from rice hull ash by supercritical carbon dioxide drying. J Supercrit Fluids. 2005;35:91–4. https://doi.org/10.1016/j.supflu.2004.12.003.
Yin W, Rubenstein DA. Biomedical applications of aerogels. In: Aegerter M, Leventis N, Koebel M, editors. Aerogels Handbook. New York, NY: Springer; 2011. p. 683–94.
Gurav JL, Jung I-K, Park H-H, Kang ES, Nadargi DY. Silica aerogel: synthesis and applications. J Nanomater. 2010;2010:1–11. https://doi.org/10.1155/2010/409310.
García-González CA, Alnaief M, Smirnova I. Polysaccharide-based aerogels—promising biodegradable carriers for drug delivery systems. Carbohydr Polym. 2011;86:1425–38. https://doi.org/10.1016/j.carbpol.2011.06.066.
Stergar J, Maver U. Review of aerogel-based materials in biomedical applications. J Solgel Sci Technol. 2016;77:738–52. https://doi.org/10.1007/s10971-016-3968-5.
Ulker Z, Erkey C. An emerging platform for drug delivery: aerogel based systems. J Control Release. 2014;177:51–63. https://doi.org/10.1016/j.jconrel.2013.12.033.
Mehling T, Smirnova I, Guenther U, Neubert R. Polysaccharide-based aerogels as drug carriers. J Non Cryst Solids. 2009;355:2472–9. https://doi.org/10.1016/j.jnoncrysol.2009.08.038.
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos. 2015;43:1823–37. https://doi.org/10.1124/dmd.115.065920.
Zhuang X, Lu C. PBPK modeling and simulation in drug research and development. Acta Pharm Sin B. 2016;6:430–40. https://doi.org/10.1016/j.apsb.2016.04.004.
Jones HM, Rowland-Yeo K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacometrics Syst Pharmacol. 2013;2:1–12. https://doi.org/10.1038/psp.2013.41.
Rowland M. Physiologically-based pharmacokinetic (PBPK) modeling and simulations principles, methods, and applications in the pharmaceutical industry. CPT Pharmacometrics Syst Pharmacol. 2013;2:e55. https://doi.org/10.1038/psp.2013.29.
Jamei M. Recent advances in development and application of physiologically-based pharmacokinetic (PBPK) models: a transition from academic curiosity to regulatory acceptance. Curr Pharmacol Rep. 2016;2:161–9. https://doi.org/10.1007/s40495-016-0059-9.
Hartmanshenn C, Scherholz M, Androulakis IP. Physiologically-based pharmacokinetic models: approaches for enabling personalized medicine. J Pharmacokinet Pharmacodyn. 2016;43:481–504. https://doi.org/10.1007/s10928-016-9492-y.
Gaohua L, Wedagedera J, Small BG, Almond L, Romero K, Hermann D, et al. Development of a multicompartment permeability-limited lung PBPK model and its application in predicting pulmonary pharmacokinetics of antituberculosis drugs. CPT Pharmacometrics Syst Pharmacol. 2015;4:605–13. https://doi.org/10.1002/psp4.12034.
Kong Y, Cai H, Xing H, Ren C, Kong D, Ning C, et al. Pulmonary delivery alters the disposition of raloxifene in rats. J Pharm Pharmacol. 2020;72:185–96. https://doi.org/10.1111/jphp.13201.
Kolli AR, Kuczaj AK, Martin F, Hayes AW, Peitsch MC, Hoeng J. Bridging inhaled aerosol dosimetry to physiologically based pharmacokinetic modeling for toxicological assessment: nicotine delivery systems and beyond. Crit Rev Toxicol. 2019;49:725–41. https://doi.org/10.1080/10408444.2019.1692780.
Hydroxychloroquinle Sulfate. The Pharmacopeia of the United States of America. Rockville, MD. 2019. Accessed 14 Sept 2022.
Mehvar R. Application of organ clearance to estimation of the in vivo hepatic extraction ratio. Curr Clin Pharmacol. 2016;11:47–52. https://doi.org/10.2174/1574884710666150817104746.
Dash RP, Veeravalli V, Thomas JA, Rosenfeld C, Mehta N, Srinivas NR. Whole blood or plasma: what is the ideal matrix for pharmacokinetic-driven drug candidate selection? Future Med Chem. 2020;13:157–71. https://doi.org/10.4155/fmc-2020-0187.
Sok V, Marzan F, Gingrich D, Aweeka F, Huang L. Development and validation of an LC-MS/MS method for determination of hydroxychloroquine, its two metabolites, and azithromycin in EDTA-treated human plasma. PLoS One. 2021;16:e0247356. https://doi.org/10.1371/journal.pone.0247356.
Soetaert K, Petzoldt T. Inverse modelling, sensitivity and monte carlo analysis in R using package FME. J Stat Softw. 2010;33:1–28. https://doi.org/10.18637/jss.v033.i03.
Obaidat R, Al-Jbour N, Al-Sou’d K, Sweidan K, Al-Remawi M, Badwan A. Some physico-chemical properties of low molecular weight chitosans and their relationship to conformation in aqueous solution. J Solution Chem. 2010;39:575–88. https://doi.org/10.1007/s10953-010-9517-x.
Alnaief M, Obaidat RM, Alsmadi MM. Preparation of hybrid alginate-chitosan aerogel as potential carriers for pulmonary drug delivery. Polymers. 2020;12:2223. https://doi.org/10.3390/polym12102223.
Alsmadi MM, Obaidat RM, Alnaief M, Albiss BA, Hailat N. Development, In vitro characterization, and in vivo toxicity evaluation of chitosan-alginate nanoporous carriers loaded with cisplatin for lung cancer treatment. AAPS PharmSciTech. 2020;21:1–12. https://doi.org/10.1208/s12249-020-01735-8.
dos Santos P, Viganó J, de Figueiredo FG, Cunha RL, Hubinger MD, Rezende CA, et al. Production of resveratrol loaded alginate aerogel: characterization, mathematical modeling, and study of impregnation. J Supercrit Fluids. 2020;163:104882. https://doi.org/10.1016/j.supflu.2020.104882.
Pishnamazi M, Hosseini S, Zabihi S, Borousan F, Hezave AZ, Marjani A, et al. Chloroquine (antimalaria medication with anti SARS-CoV activity) solubility in supercritical carbon dioxide. J Mol Liq. 2021;322:114539. https://doi.org/10.1016/j.molliq.2020.114539.
Obaidat RM, Tashtoush BM, Bayan MF, Al Bustami RT, Alnaief M. Drying using supercritical fluid technology as a potential method for preparation of chitosan aerogel microparticles. AAPS PharmSciTech. 2015;16:1235–44. https://doi.org/10.1208/s12249-015-0312-2.
Alnaief M, Obaidat R, Mashaqbeh H. Effect of processing parameters on preparation of carrageenan aerogel microparticles. Carbohydr Polym. 2018;180:264–75. https://doi.org/10.1016/j.carbpol.2017.10.038.
Obaidat R, Aleih H, Mashaqbeh H, Altaani B, Alsmadi MTM, Alnaief M. Development and evaluation of cocoa butter taste masked ibuprofen using supercritical carbon dioxide. AAPS PharmSciTech. 2021;22:106. https://doi.org/10.1208/s12249-021-01962-7.
Unagolla JM, Jayasuriya AC. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur J Pharm Sci. 2018;114:199–209. https://doi.org/10.1016/j.ejps.2017.12.012.
He Y, Xu Y, Huang Y, Quang H, Xia X, Zhao H, et al. Redox sensitive nano-capsules self-assembled from hyaluronic acid-hydroxychloroquine conjugates for CD44-targeted delivery of hydroxychloroquine to combat breast cancer metastasis in vitro and in vivo. Colloids Surf B Biointerfaces. 2022;210:112249. https://doi.org/10.1016/j.colsurfb.2021.112249.
Yin S, Xia C, Wang Y, Wan D, Rao J, Tang X, et al. Dual receptor recognizing liposomes containing paclitaxel and hydroxychloroquine for primary and metastatic melanoma treatment via autophagy-dependent and independent pathways. J Control Release. 2018;288:148–60. https://doi.org/10.1016/j.jconrel.2018.08.015.
Moore JW, Flanner HH. Mathematical comparison of dissolution profiles. Pharm Technol. 1996;20:64–74.
Tai T-T, Wu T-J, Wu H-D, Tsai Y-C, Wang H-T, Wang A-M, Shih S-F, Chen Y-C. A strategy to treat COVID-19 disease with targeted delivery of inhalable liposomal hydroxychloroquine: a pre-clinical pharmacokinetic study. Clin Transla Sci. 2020:14. https://doi.org/10.1111/cts.12923.
Emami J, Pasutto FM, Jamali F. Effect of experimental diabetes mellitus and arthritis on the pharmacokinetics of hydroxychloroquine enantiomers in rats. Pharm Res. 1998;15:897–903. https://doi.org/10.1023/A:1011928732588.
McGuill MW, Rowan AN. Biological effects of blood loss: implications for sampling volumes and techniques. ILAR J. 1989;31:5–20. https://doi.org/10.1093/ilar.31.4.5.
Chhonker YS, Sleightholm RL, Li J, Oupický D, Murry DJ. Simultaneous quantitation of hydroxychloroquine and its metabolites in mouse blood and tissues using LC–ESI–MS/MS: an application for pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1072:320–7. https://doi.org/10.1016/j.jchromb.2017.11.026.
Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Meth Prog Biomed. 2010;99:306–14. https://doi.org/10.1016/j.cmpb.2010.01.007.
Gabrielsson J, Weiner D. Non-compartmental analysis. In: Reisfeld B, Mayeno A, editors. Computational toxicology methods in molecular biology. Totowa, NJ: Humana Press; 2012. p. 377–89.
Amer MG, Mohamed NM. Potential use of quercetin as protective agent against hydroxychloroquine induced cardiotoxicity. J Biomed Res Environ Sci. 2021;2(185-92) https://doi.org/10.37871/jbres1208.
Kim K, Chini N, Fairchild DG, Engle SK, Reagan WJ, Summers SD, et al. Evaluation of cardiac toxicity biomarkers in rats from different laboratories. Toxicol Pathol. 2016;44:1072–83. https://doi.org/10.1177/0192623316668276.
Roche. CKMB Creatine Kinase-MB. 2022. https://diagnostics.roche.com/global/en/products/product-category/molecular-diagnostics.html. Accessed 7 Nov 2022.
Roche. LDHI2 Lactate dehydrogenase acc. to IFCC ver.2. 2019. https://labogids.sintmaria.be/sites/default/files/files/ldhi2_2019-07_v12.pdf. Accessed 7 Nov 2022.
Roshan VD, Assali M, Moghaddam AH, Hosseinzadeh M, Myers J. Exercise training and antioxidants: effects on rat heart tissue exposed to lead acetate. Int J Toxicol. 2011;30:190–6. https://doi.org/10.1177/1091581810392809.
Srikanth G, Prakash P, Tripathy N, Dikshit M, Nityanand S. Establishment of a rat model of myocardial infarction with a high survival rate: a suitable model for evaluation of efficacy of stem cell therapy. J Stem Cells Regen Med. 2009;5:30–6. https://doi.org/10.46582/jsrm.0501006.
Karthikeyan K, Bai BR, Devaraj SN. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats. Int J Cardiol. 2007;115:326–33. https://doi.org/10.1016/j.ijcard.2006.03.016.
Swift ML. GraphPad Prism, Data analysis, and scientific graphing. J Chem Inf Comput Sci. 1997;37:411–2. https://doi.org/10.1021/ci960402j.
Razali NM, Wah YB. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. JOSMA. 2011;2:21–33.
Mukherjee B. Statistics in pharmacokinetics. Pharmacokinetics: basics to applications. Singapore: Springer; 2022. p. 199–226.
McKnight PE, Najab J. Mann-Whitney U Test. The Corsini encyclopedia of psychology. 2010: 1 https://doi.org/10.1002/9780470479216.corpsy0524.
Thelen K, Coboeken K, Willmann S, Burghaus R, Dressman JB, Lippert J. Evolution of a detailed physiological model to simulate the gastrointestinal transit and absorption process in humans, part 1: oral solutions. J Pharm Sci. 2011;100:5324–45. https://doi.org/10.1002/jps.22726.
Tai T-T, Wu T-J, Wu H-D, Tsai Y-C, Wang H-T, Wang A-M, et al. A strategy to treat COVID-19 disease with targeted delivery of inhalable liposomal hydroxychloroquine: a preclinical pharmacokinetic study. Clin Transl Sci. 2021;14:132–6. https://doi.org/10.1111/cts.12923.
Simulations Plus Inc. GastroPlus Manual. 2020. www.simulations-plus.com/wp-content/uploads/GastroPlus-9.8-Release-Notes.pdf. Accessed 20 Dec 2022.
Margolskee A, Darwich AS, Pepin X, Aarons L, Galetin A, Rostami-Hodjegan A, et al. IMI–oral biopharmaceutics tools project–evaluation of bottom-up PBPK prediction success part 2: an introduction to the simulation exercise and overview of results. Eur J Pharm Sci. 2017;96:610–25. https://doi.org/10.1016/j.ejps.2016.10.036.
Zambuzi GC, Camargos CHM, Ferreira MP, Rezende CA, de Freitas O, Francisco KR. Modulating the controlled release of hydroxychloroquine mobilized on pectin films through film-forming pH and incorporation of nanocellulose. Carbohydrate polymers. 2021;2:100140. https://doi.org/10.1016/j.carpta.2021.100140.
Moraes ANF, Silva LAD, de Oliveira MA, de Oliveira EM, Nascimento TL, Lima EM, et al. Compatibility study of hydroxychloroquine sulfate with pharmaceutical excipients using thermal and nonthermal techniques for the development of hard capsules. J Therm Anal Calorim. 2020;140:2283–92. https://doi.org/10.1007/s10973-019-08953-8.
Barboza F, Vecchia DD, Tagliari MP, Silva MAS, Stulzer HK. Differential scanning calorimetry as a screening technique in compatibility studies of acyclovir extended release formulations. Pharm Chem J. 2009;43:363–8. https://doi.org/10.1007/s11094-009-0304-1.
Arianto A, Bangun H, Harahap U, Ilyas S. Effect of alginate chitosan ratio on the swelling, mucoadhesive, and release of ranitidine from spherical matrices of alginate-chitosan. Int J Pharmtech Res. 2015;8:653–65.
Armutcu C, Pişkin S. Evaluation of controlled hydroxychloroquine releasing performance from calcium-alginate beads. HJSE. 2021;8:255–63. https://doi.org/10.17350/HJSE19030000236.
Treasa MS, Premakumari J. Characterisation and solubility studies of quinine sulphate and hydroxychloroquine sulphate inclusion complexes with α–cyclodextrin. IOSR j appl chem. 2018;11:24–34. https://doi.org/10.9790/5736-1111012434.
Einfalt T, Detampel P, Häussinger D, Casper J, Meier CR, Puchkov M, et al. Hydroxychloroquine immediate release tablets: formulation and evaluation of a solid dosage form. Biological and Medicinal. Chem. 2021; https://doi.org/10.26434/chemrxiv.14170508.v1.
Dubey R, Bajpai J, Bajpai AK. Chitosan-alginate nanoparticles (CANPs) as potential nanosorbent for removal of Hg (II) ions. Environ Nanotechnol Monit Manag. 2016;6:32–44. https://doi.org/10.1016/j.enmm.2016.06.008.
Ben-Jebria A, Chen D, Eskew ML, Vanbever R, Langer R, Edwards DA. Large porous particles for sustained protection from carbachol-induced bronchoconstriction in guinea pigs. Pharm Res. 1999;16:555–61. https://doi.org/10.1023/A:1018879331061.
Saha T, Quiñones-Mateu ME, Das SC. Inhaled therapy for COVID-19: considerations of drugs, formulations and devices. Int J Pharm. 2022;624:122042. https://doi.org/10.1016/j.ijpharm.2022.122042.
Bae JY, Lee GE, Park H, Cho J, Kim J, Lee J, et al. Antiviral efficacy of pralatrexate against SARS-CoV-2. Biomol Ther (Seoul). 2021;29:268–72. https://doi.org/10.4062/biomolther.2021.032.
Tahara K, Sakai T, Yamamoto H, Takeuchi H, Hirashima N, Kawashima Y. Improved cellular uptake of chitosan-modified PLGA nanospheres by A549 cells. Int J Pharm. 2009;382:198–204. https://doi.org/10.1016/j.ijpharm.2009.07.023.
Takka S, Gürel A. Evaluation of chitosan/alginate beads using experimental design: formulation and in vitro characterization. AAPS PharmSciTech. 2010;11:460–6. https://doi.org/10.1208/s12249-010-9406-z.
Jia M, Li Z-B, Chu H-T, Li L, Chen K-Y. Alginate-chitosan microspheres for controlled drug delivery of diltiazem hydrochloride in cardiac diseases. J Biomater Tissue Eng. 2015;5:246–51. https://doi.org/10.1166/jbt.2015.1299.
Chan G, Mooney DJ. Ca2+ released from calcium alginate gels can promote inflammatory responses in vitro and in vivo. Acta Biomater. 2013;9:9281–91. https://doi.org/10.1016/j.actbio.2013.08.002.
USFDA. Physiologically based pharmacokinetic analyses-format and content guidance for industry. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/physiologically-based-pharmacokinetic-analyses-format-and-content-guidance-industry. Accessed 26 Oct 2022.
Ono C, Hsyu P-H, Abbas R, Loi C-M, Yamazaki S. Application of physiologically based pharmacokinetic modeling to the understanding of bosutinib pharmacokinetics: prediction of drug–drug and drug–disease interactions. Drug Metab Dispos. 2017;45:390–8. https://doi.org/10.1124/dmd.116.074450.
Obaidat RM, Tashtoush BM, Awad AA, Al Bustami RT. Using supercritical fluid technology (SFT) in preparation of tacrolimus solid dispersions. AAPS PharmSciTech. 2017;18:481–93. https://doi.org/10.1208/s12249-016-0492-4.
García-González C, Uy J, Alnaief M, Smirnova I. Preparation of tailor-made starch-based aerogel microspheres by the emulsion-gelation method. Carbohydr Polym. 2012;88:1378–86. https://doi.org/10.1016/j.carbpol.2012.02.023.
Marin MA, Mallepally RR, McHugh MA. Silk fibroin aerogels for drug delivery applications. J Supercrit Fluids. 2014;91:84–9. https://doi.org/10.1016/j.supflu.2014.04.014.
Ulker Z, Erkey C. An advantageous technique to load drugs into aerogels: gas antisolvent crystallization inside the pores. J Supercrit Fluids. 2017;120:310–9. https://doi.org/10.1016/j.supflu.2016.05.033.
Smola M, Vandamme T, Sokolowski A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int J Nanomedicine. 2008;3:1–19.
Menon JU, Ravikumar P, Pise A, Gyawali D, Hsia CC, Nguyen KT. Polymeric nanoparticles for pulmonary protein and DNA delivery. Acta Biomater. 2014;10:2643–52. https://doi.org/10.1016/j.actbio.2014.01.033.
George JM, Mathew B. Cyclodextrin-mediated gold nanoparticles as multisensing probe for the selective detection of hydroxychloroquine drug. Korean J Chem Eng. 2021;38:624–34. https://doi.org/10.1007/s11814-020-0719-7.
Reddy SG. Controlled release studies of hydroxychloroquine sulphate (Hcq) drug-using biodegradable polymeric sodium alginate and lignosulphonic acid blends. Rasayan J Chem. 2021;14:2209–15.
Martins AF, Facchi SP, Monteiro JP, Nocchi SR, Silva CTP, Nakamura CV, et al. Preparation and cytotoxicity of N, N, N-trimethyl chitosan/alginate beads containing gold nanoparticles. Int J Biol Macromol. 2015;72:466–71. https://doi.org/10.1016/j.ijbiomac.2014.08.020.
Shoaib MH, Tazeen J, Merchant HA, Yousuf RI. Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak J Pharm Sci. 2006;19:119–24.
Singhvi G, Singh M. In-vitro drug release characterization models. Int J Pharm Stud Res. 2011;2:77–84.
Nie S, Hsiao WLW, Pan W, Yang Z. Thermoreversible Pluronic® F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: in vitro drug release, cell cytotoxicity, and uptake studies. Int J Nanomedicine. 2011;6:151–66. https://doi.org/10.2147/IJN.S15057.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. https://doi.org/10.1016/0378-5173(83)90064-9.
Gill KK, Nazzal S, Kaddoumi A. Paclitaxel loaded PEG5000–DSPE micelles as pulmonary delivery platform: formulation characterization, tissue distribution, plasma pharmacokinetics, and toxicological evaluation. Eur J Pharm Biopharm. 2011;79:276–84. https://doi.org/10.1016/j.ejpb.2011.04.017.
Dobrev ID, Reddy MB, Plotzke KP, Varaprath S, McNett DA, Durham J, et al. Closed-chamber inhalation pharmacokinetic studies with hexamethyldisiloxane in the rat. Inhal Toxicol. 2003;15:589–617. https://doi.org/10.1080/08958370390205083.
Mitchell J, Dolovich MB. Clinically relevant test methods to establish in vitro equivalence for spacers and valved holding chambers used with pressurized metered dose inhalers (pMDIs). J Aerosol Med Pulm Drug Deliv. 2012;25:217–42. https://doi.org/10.1089/jamp.2011.0933.
Gonçalves VSS, Gurikov P, Poejo J, Matias AA, Heinrich S, Duarte CMM, et al. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur J Pharm Biopharm. 2016;107:160–70. https://doi.org/10.1016/j.ejpb.2016.07.003.
Menshutina N, Majouga A, Uvarova A, Lovskaya D, Tsygankov P, Mochalova M, et al. Chitosan aerogel particles as nasal drug delivery systems. Gels. 2022;8:796. https://doi.org/10.3390/gels8120796.
Soorbaghi FP, Isanejad M, Salatin S, Ghorbani M, Jafari S, Derakhshankhah H. Bioaerogels: synthesis approaches, cellular uptake, and the biomedical applications. Biomed Pharmacother. 2019;111:964–75. https://doi.org/10.1016/j.biopha.2019.01.014.
Ong HX, Benaouda F, Traini D, Cipolla D, Gonda I, Bebawy M, et al. In vitro and ex vivo methods predict the enhanced lung residence time of liposomal ciprofloxacin formulations for nebulisation. Eur J Pharm Biopharm. 2014;86:83–9. https://doi.org/10.1016/j.ejpb.2013.06.024.
Brillault J, Tewes F. Control of the lung residence time of highly permeable molecules after nebulization: example of the fluoroquinolones. Pharmaceutics. 2020;12:387. https://doi.org/10.3390/pharmaceutics12040387.
Borghardt JM, Kloft C, Sharma A. Inhaled therapy in respiratory disease: the complex interplay of pulmonary kinetic processes. Can Respir J. 2018; 2018. https://doi.org/10.1155/2018/2732017.
Wang H, Xu Y, Zhou X. Docetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: in vitro and in vivo evaluation. Int J Mol Sci. 2014;15:3519–32. https://doi.org/10.3390/ijms15033519.
Zinellu A, Sotgia S, Fois AG, Mangoni AA. Serum CK-MB, COVID-19 severity and mortality: an updated systematic review and meta-analysis with meta-regression. Adv Med Sci. 2021;66:304–14. https://doi.org/10.1016/j.advms.2021.07.001.
Mohamed HE, El-Swefy SE, Hagar HH. The protective effect of glutathione administration on adriamycin-induced acute cardiac toxicity in rats. Pharmacol Res. 2000;42:115–21. https://doi.org/10.1006/phrs.1999.0630.
Nix DE, Goodwin SD, Peloquin CA, Rotella DL, Schentag J. Antibiotic tissue penetration and its relevance: models of tissue penetration and their meaning. Antimicrob Agents Chemother. 1991;35:1947–52. https://doi.org/10.1128/AAC.35.10.1947.
Yamazaki K, Ogura S, Ishizaka A, Oh-hara T, Nishimura M. Bronchoscopic microsampling method for measuring drug concentration in epithelial lining fluid. Am J Respir Crit Care Med. 2003;168:1304–7. https://doi.org/10.1164/rccm.200301-111OC.
Alsmadi MM. The investigation of the complex population-drug-drug interaction between ritonavir-boosted lopinavir and chloroquine or ivermectin using physiologically-based pharmacokinetic modeling. Drug Metab Pers Ther. 2022;38:87–105. https://doi.org/10.1515/dmpt-2022-0130.
Harcourt J, Tamin A, Lu X, Kamili S, Sakthivel SK, Murray J, et al. Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease. United States. Emerg Infect Dis. 2020;26:1266–73. https://doi.org/10.3201/eid2606.200516.
Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. https://doi.org/10.1126/science.abc1932.
Shuai H, Chan JF-W, Hu B, Chai Y, Yuen TT-T, Yin F, et al. Attenuated replication and pathogenicity of SARS-CoV-2 B. 1.1. 529 Omicron. Nature. 2022:1. https://doi.org/10.1038/s41586-022-04442-5.
Abdelnabi R, Foo CS, Zhang X, Lemmens V, Maes P, Slechten B, et al. The omicron (B. 1.1. 529) SARS-CoV-2 variant of concern does not readily infect Syrian hamsters. Antiviral Res. 2022;198:105253. https://doi.org/10.1016/j.antiviral.2022.105253.
Mslati H, Gentile F, Perez C, Cherkasov A. Comprehensive consensus analysis of SARS-CoV-2 drug repurposing campaigns. J Chem Inf Model. 2021;61:3771–88. https://doi.org/10.1021/acs.jcim.1c00384.
Wei Y, Nygard GA, Ellertson SL, Khalil SK. Stereoselective disposition of hydroxychloroquine and its metabolites in rats. Chirality. 1995;7:598–604. https://doi.org/10.1002/chir.530070807.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94:1259–76. https://doi.org/10.1002/jps.20322.
Tai W, Chow MYT, Chang RYK, Tang P, Gonda I, MacArthur RB, et al. Nebulised isotonic hydroxychloroquine aerosols for potential treatment of COVID-19. Pharmaceutics. 2021;13:1260. https://doi.org/10.3390/pharmaceutics13081260.
Miller NA, Graves RH, Edwards CD, Amour A, Taylor E, Robb O, et al. Physiologically based pharmacokinetic modelling of inhaled nemiralisib: mechanistic components for pulmonary absorption, systemic distribution, and oral absorption. Clin Pharmacokinet. 2022;61:281–93. https://doi.org/10.1007/s40262-021-01066-2.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12:263–71. https://doi.org/10.1208/s12248-010-9185-1.
Acknowledgements
The authors acknowledge Jordan University of Science and Technology (Irbid, Jordan) for all facilities and support provided. Also, the authors acknowledge ACDIMA Biocenter (Amman, Jordan) for developing the assay method and analyzing hydroxychloroquine concentration in blood samples collected from rats. Finally, the authors acknowledge Simulations Plus Inc. (Lancaster, CA, U.S.A.) for providing an academic license of GastroPlus® used to analyze the hydroxychloroquine pharmacokinetic data measured in the current study and that reported in the literature used to verify the model.
Funding
This project was funded by a local fund (grant no.: 53/2022) provided by Jordan University of Science and Technology (Irbid, Jordan).
Author information
Authors and Affiliations
Contributions
• Alsmadi, Jaradat, Obiadat, and Alnaief made substantial contributions to the conception and design of the work.
• Alsmadi, Jaradat, Obiadat, and Tayyem had substantial contributions to the acquisition of the data.
• Alsmadi, Jaradat, Obiadat, Alnaief, and Idkaidek had substantial contributions to the analysis and interpretation of data for the work.
• Alsmadi, Jaradat, Obiadat, and Idkaidek had substantial contributions to drafting the work and revising it critically for important intellectual content.
• All authors approved and agreed to be accountable for all aspects of the final version to be published and ensured that the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 201 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alsmadi, M.M., Jaradat, M.M., Obaidat, R.M. et al. The In Vitro, In Vivo, and PBPK Evaluation of a Novel Lung-Targeted Cardiac-Safe Hydroxychloroquine Inhalation Aerogel. AAPS PharmSciTech 24, 172 (2023). https://doi.org/10.1208/s12249-023-02627-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02627-3