Abstract
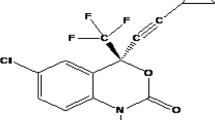
The present research work attempted to improve the oral bioavailability of the antiviral drug Efavirenz (EFV) using a pharmaceutical cocrystallization technique. EFV comes under BCS-II and has extremely low water solubility, and results in low oral bioavailability. EFV and nicotinamide (NICO) were selected in a (1:1) stoichiometric ratio and efavirenz nicotinamide cocrystal (ENCOC) was prepared through the liquid-assisted grinding method (LAG). The confirmation of the formation of a new solid phase was done through spectroscopic techniques like Fourier transmission infrared (FTIR), Raman, and 13C solid-state nuclear magnetic resonance (13C ssNMR). Thermal techniques like differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and hot stage microscopy (HSM) illustrated the thermal behavior and melting patterns of ENCOC, EFV, and NICO. The X-ray powder diffraction (XRPD) confirms the formation of a new crystalline phase in ENCOC. The Morphology was determined through scanning electron microscopy (FESEM). The results of saturated solubility studies and in vitro drug release studies exhibited 8.9-fold enhancement in solubility and 2.56-fold enhancement in percentage cumulative drug release. The percentage drug content of ENCOC was found higher than 97% and cocrystal exhibits excellent accelerated stability. The oral bioavailability of EFV (Cmax, 799.08 ng/mL) exhibits significant enhancement after cocrystallization (Cmax, 5597.09 ng/mL) than EFV and Efcure®-200 tablet (2896.21 ng/mL). The current work investigates the scalable and cost-effective method for enhancement of physicochemical stability, solubility, and oral bioavailability of an antiviral agent EFV.








Similar content being viewed by others
References
WHO. WHO fact sheet HIV 2022 [Internet]. 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/hiv-aids. Accessed 02/03/2021.
Savjani JK, Pathak C. Improvement of physicochemical parameters of acyclovir using cocrystallization approach. Brazilian J Pharm Sci [Internet]. 2016;52:727–34. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1984-82502016000400727&lng=en&tlng=en.
Ngilirabanga JB, Rosa PP, Aucamp M, Kippie Y, Samsodien H. Dual-drug co-crystal synthesis for synergistic in vitro effect of three key first-line antiretroviral drugs. J Drug Deliv Sci Technol. 2020;60.
Saeed AM, Schmidt JM, Munasinghe WP, Vallabh BK, Jarvis MF, Morris JB, et al. Comparative bioavailability of two formulations of biopharmaceutical classification system (BCS) class IV drugs: a case study of lopinavir/ritonavir. J Pharm Sci [Internet]. 2021;110:3963–8.
Rehman S, Nabi B, Fazil M, Khan S, Bari NK, Singh R, et al. Role of P-glycoprotein inhibitors in the bioavailability enhancement of solid dispersion of darunavir. Biomed Res Int [Internet]. Hindawi Limited; 2017 [cited 2021 Feb 3];2017:1–17. Available from: https://www.hindawi.com/journals/bmri/2017/8274927/.
Chaudhari KR, Savjani JK, Savjani KT, Shah H. Improved pharmaceutical properties of ritonavir through co-crystallization approach with liquid-assisted grinding method. Drug Dev Ind Pharm [Internet]. 2022;1–10. Available from: https://www.tandfonline.com/doi/full/10.1080/03639045.2022.2042553.
Cysewski P, Przybyłek M. Selection of effective cocrystals former for dissolution rate improvement of active pharmaceutical ingredients based on lipoaffinity index. Eur J Pharm Sci [Internet]. 2017;107:87–96. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0928098717304037.
Takahashi H, Iwama S, Clevers S, Veesler S, Coquerel G, Tsue H, et al. In situ observation of polymorphic transition during crystallization of organic compounds showing preferential enrichment by means of temperature-controlled video-microscopy and time-resolved X-ray powder diffraction. Cryst Growth Des [Internet]. 2017;17:671–6.
Zolotov SA, Demina NB, Zolotova AS, Shevlyagina N V., Buzanov GA, Retivov VM, et al. Development of novel darunavir amorphous solid dispersions with mesoporous carriers. Eur J Pharm Sci [Internet]. Elsevier B.V.; 2021;159:105700. Available from: https://doi.org/10.1016/j.ejps.2021.105700.
Pawar J, Tayade A, Gangurde A, Moravkar K, Amin P. Solubility and dissolution enhancement of efavirenz hot melt extruded amorphous solid dispersions using combination of polymeric blends: A QbD approach. Eur J Pharm Sci [Internet]. 2016;88:37–49. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0928098716301026.
Mazumder S, Dewangan AK, Pavurala N. Enhanced dissolution of poorly soluble antiviral drugs from nanoparticles of cellulose acetate based solid dispersion matrices. Asian J Pharm Sci [Internet]. Elsevier B.V.; 2017;12:532–41. Available from: https://doi.org/10.1016/j.ajps.2017.07.002.
Dahiya S, Savjani K, Savjani J. Development, characterization, and optimization of a novel abiraterone acetate formulation to improve biopharmaceutical attributes aided by pharmacokinetic modelling. AAPS PharmSciTech [Internet]. 2022;23:4. Available from: https://link.springer.com/10.1208/s12249-021-02168-7.
Rao MRP, Chaudhari J, Trotta F, Caldera F. Investigation of cyclodextrin-based nanosponges for solubility and bioavailability enhancement of rilpivirine. AAPS PharmSciTech [Internet]. Springer New York LLC; 2018 [cited 2021 Feb 3];19:2358–69. Available from: https://link.springer.com/article/10.1208/s12249-018-1064-6.
Kagade S, Savjani J, Yadav D, Gurav P. Isoxsuprine hydrochloride loaded cellulose acetate phthalate microsponge drug delivery system: design and evaluation. Indian J Pharm Educ Res [Internet]. 2020;54:s173–81. Available from: http://ijper.org/article/1167.
Chaudhari KS, Akamanchi KG. Novel bicephalous heterolipid based self-microemulsifying drug delivery system for solubility and bioavailability enhancement of efavirenz. Int J Pharm [Internet]. Elsevier B.V.; 2019 [cited 2021 Feb 3];560:205–18. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378517319301061.
Center of Drug Evaluation and Research. Regulatory classification of pharmaceutical co-crystals, guidance for industry. Food Drug Adm US Dep Heal Hum Serv [Internet]. 2018;1–4. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm%0A, https://www.fda.gov/media/81824/download. Accessed 02/03/2022.
Rodrigues M, Baptista B, Lopes JA, Sarraguça MC. Pharmaceutical cocrystallization techniques. Advances and challenges. Int J Pharm [Internet]. Elsevier; 2018;547:404–20. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378517318304216.
Chavan RB, Thipparaboina R, Yadav B, Shastri NR. Continuous manufacturing of co-crystals: challenges and prospects. Drug Deliv. Transl. Res. Drug Delivery and Translational Research; 2018. p. 1726–39.
Malamatari M, Ross SA, Douroumis D, Velaga SP. Experimental cocrystal screening and solution based scale-up cocrystallization methods. Adv Drug Deliv Rev [Internet]. Elsevier B.V.; 2017;117:162–77. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0169409X17301540.
Kumar Bandaru R, Rout SR, Kenguva G, Gorain B, Alhakamy NA, Kesharwani P, et al. Recent advances in pharmaceutical cocrystals: from bench to market. Front Pharmacol. 2021;12:1–16.
Kavanagh ON, Croker DM, Walker GM, Zaworotko MJ. Pharmaceutical cocrystals: from serendipity to design to application. Drug Discov Today [Internet]. 2019;24:796–804. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1359644618303751.
Adkins JC, Noble S. Efavirenz. Drugs [Internet]. 1998;56:1055–64. Available from: http://link.springer.com/10.2165/00003495-199856060-00014.
Efavirenz | C14H9ClF3NO2 - PubChem [Internet].Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Efavirenz#section=Solubility. Accessed 03/02/2021.
Rao MRP, Bhutada K, Kaushal P. Taste evaluation by electronic tongue and bioavailability enhancement of efavirenz. AAPS PharmSciTech. AAPS PharmSciTech; 2019;20.
Fandaruff C, Rauber GS, Araya-Sibaja AM, Pereira RN, de Campos CEM, Rocha HVA, et al. Polymorphism of anti-HIV drug efavirenz: investigations on thermodynamic and dissolution properties. Cryst Growth Des [Internet]. 2014;14:4968–75. Available from: https://pubs.acs.org/doi/10.1021/cg500509c.
Chaves Júnior JV, dos Santos JAB, Lins TB, de Araújo Batista RS, de Lima Neto SA, de Santana Oliveira A, et al. A new ferulic acid–nicotinamide cocrystal with improved solubility and dissolution performance. J Pharm Sci [Internet]. 2020;109:1330–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S002235491930807X.
Park B, Yoon W, Yun J, Ban E, Yun H, Kim A. Emodin-nicotinamide (1:2) cocrystal identified by thermal screening to improve emodin solubility. Int J Pharm [Internet]. 2019;557:26–35. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378517318309372.
Nugrahani I, Fisandra F, Horikawa A, Uekusa H. New sodium mefenamate – nicotinamide multicomponent crystal development to modulate solubility and dissolution: preparation, structural, and performance study. J Pharm Sci [Internet]. 2021;110:3246–60. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022354921002872.
Wang L, Yan Y, Zhang X, Zhou X. Novel pharmaceutical cocrystal of lenalidomide with nicotinamide: structural design, evaluation, and thermal phase transition study. Int J Pharm [Internet]. 2022;613:121394. Available from: https://linkinghub.elsevier.com/retrieve/pii/S037851732101200X.
Aramanda SK, Khanna S, Salapaka SK, Chattopadhyay K, Choudhury A. Crystallographic and morphological evidence of solid–solid interfacial energy anisotropy in the Sn-Zn eutectic system. Metall Mater Trans A [Internet]. 2020;51:6387–405. Available from: http://link.springer.com/10.1007/s11661-020-06007-5.
Dr. Friedrich Menges. Spectragryph - optical spectroscopy software: 1.12.15 [Internet]. 2020 [cited 2022 Jun 2]. Available from: https://www.effemm2.de/spectragryph/license_copy.html
Match! - Phase Analysis using Powder Diffraction, Version 3.x, Crystal Impact - Dr. H. Putz & Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany, https://www.crystalimpact.de/match.
OriginLab Corporation. OriginPro, Version 2022b. OriginLab Corporation, Northampton, MA, USA. Northampton, MA, USA, 2022.
Holzwarth U, Gibson N. The Scherrer equation versus the “Debye-Scherrer equation.” Nat. Nanotechnol. 2011. p. 534.
ICH Expert Working Group. Ich Harmonised Guideline - Impurities: Guideline for Residual Solvents Q3C(R8). Int Counc Harmon Tech Requir Pharm Hum Use. 2021;1–43.
Pawar Jaywant N, Amin Purnima D. Development of efavirenz cocrystals from stoichiometric solutions by spray drying technology. Mater Today Proc [Internet]. Elsevier Ltd; 2016;3:1742–51. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214785316300864.
Bedor DCG, Bedor NCTC, Neto JGP, José de Alencar Danda L, de Oliveira FM, de Oliveira GHO, et al. Characterization, in vitro dissolution, and pharmacokinetics of different batches of efavirenz raw materials. Drug Dev Ind Pharm [Internet]. 2021;47:725–34. Available from: https://www.tandfonline.com/doi/full/10.1080/03639045.2021.1934860.
Wardhana YW, Aisyah EN, Sopyan I, Rusdiana T. In vitro solubility and release profile correlation with pka value of efavirenz polymorphs. Dissolution Technol. 2021;28:14–22.
USFDA. Drug Dissolution method Database [Internet]. 2006. Available from: https://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_getalldata.cfm. Accessed 2/03/2021.
Wang L, Yan Y, Zhang X, Zhou X. Novel pharmaceutical cocrystal of lenalidomide with nicotinamide: structural design, evaluation, and thermal phase transition study. Int J Pharm [Internet]. Elsevier B.V.; 2022;613:121394. Available from: https://doi.org/10.1016/j.ijpharm.2021.121394.
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE. Requirements for registration of pharmaceuticals for human stability testing of. 2003;Q1A(R2):1–18.
Al-kazemi R, Al-basarah Y, Nada A. Atorvastatin cocrystals : tablet formulation and stability. Asian J Pharm. 2020;14:578–95.
Nechipadappu SK, Reddy IR, Tarafder K, Trivedi DR. Salt/cocrystal of anti-fibrinolytic hemostatic drug tranexamic acid: structural, DFT, and stability study of salt/cocrystal with GRAS molecules. Cryst Growth Des. 2019;19:347–61.
Yadav AV, Dabke AP, Shete AS. Crystal engineering to improve physicochemical properties of mefloquine hydrochloride. Drug Dev Ind Pharm. 2010;36:1036–45.
Shi X, Wang C, Chen Q, Shen S, Song S, Zhou X. Improving physicochemical properties of Ibrutinib with cocrystal strategy based on structures and natures of the carboxylic acid co-formers. J Drug Deliv Sci Technol [Internet]. Elsevier B.V.; 2021;63:102554. Available from: https://doi.org/10.1016/j.jddst.2021.102554.
Zhou F, Zhou J, Zhang H, Tong HHY, Nie J, Li L, et al. Structure determination and in vitro/vivo study on carbamazepine-naringenin (1:1) cocrystal. J Drug Deliv Sci Technol [Internet]. Elsevier; 2019;54:101244. Available from: https://doi.org/10.1016/j.jddst.2019.101244.
Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed [Internet]. 2010;99:306–14. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0169260710000209.
Rodrigues M, Lopes J, Guedes A, Sarraguça J, Sarraguça M. Considerations on high-throughput cocrystals screening by ultrasound assisted cocrystallization and vibrational spectroscopy. Spectrochim Acta - Part A Mol Biomol Spectrosc [Internet]. Elsevier B.V.; 2020;229:117876. Available from: https://doi.org/10.1016/j.saa.2019.117876.
Liu Y, An C, Luo J, Wang J. High-density HNIW/TNT cocrystal synthesized using a green chemical method. Acta Crystallogr Sect B Struct Sci Cryst Eng Mater. 2018;74:385–93.
Bhandaru JS, Malothu N, Akkinepally RR. Characterization and solubility studies of pharmaceutical cocrystals of eprosartan mesylate. Cryst Growth Des. 2015;15:1173–9.
Xiao L, Guo S, Su H, Gou B, Liu Q, Hao G, et al. Preparation and characteristics of a novel PETN/TKX-50 co-crystal by a solvent/non-solvent method. RSC Adv Royal Society of Chemistry. 2019;9:9204–10.
Manin AN, Voronin AP, Drozd K V., Manin NG, Bauer-Brandl A, Perlovich GL. Cocrystal screening of hydroxybenzamides with benzoic acid derivatives: a comparative study of thermal and solution-based methods. Eur J Pharm Sci [Internet]. Elsevier B.V.; 2014;65:56–64. Available from: https://doi.org/10.1016/j.ejps.2014.09.003.
Saganowska P, Wesolowski M. DSC as a screening tool for rapid co-crystal detection in binary mixtures of benzodiazepines with co-formers. J Therm Anal Calorim [Internet]. Springer Netherlands; 2018;133:785–95. Available from: https://doi.org/10.1007/s10973-017-6858-3.
Gohel SK V., Sanphui P, Singh GP, Bhat K, Prakash M. Lower melting pharmaceutical cocrystals of metaxalone with carboxamide functionalities. J Mol Struct. Elsevier B.V.; 2019;1178:479–90.
Perlovich G. Melting points of one- and two-component molecular crystals as effective characteristics for rational design of pharmaceutical systems. Acta Crystallogr Sect B Struct Sci Cryst Eng Mater [Internet]. 2020;76:696–706. Available from: https://scripts.iucr.org/cgi-bin/paper?S2052520620007362.
Zheng K, Li A, Wu W, Qian S, Liu B, Pang Q. Preparation, characterization, in vitro and in vivo evaluation of metronidazole–gallic acid cocrystal: a combined experimental and theoretical investigation. J Mol Struct [Internet]. 2019;1197:727–35. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022286019309469.
Ul Islam N, Khan E, Naveed Umar M, Shah A, Zahoor M, Ullah R, et al. Enhancing dissolution rate and antibacterial efficiency of azithromycin through drug-drug cocrystals with paracetamol. Antibiotics [Internet]. 2021;10:939. Available from: https://www.mdpi.com/2079-6382/10/8/939.
Manin AN, Voronin AP, Drozd K V., Manin NG, Bauer-Brandl A, Perlovich GL. Cocrystal screening of hydroxybenzamides with benzoic acid derivatives: a comparative study of thermal and solution-based methods. Eur J Pharm Sci [Internet]. 2014;65:56–64. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0928098714003443.
Chadha K, Karan M, Chadha R, Bhalla Y, Vasisht K. Is failure of cocrystallization actually a failure? Eutectic formation in cocrystal screening of hesperetin. J Pharm Sci [Internet]. 2017;106:2026–36. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022354917302745.
Macrae CF, Sovago I, Cottrell SJ, Galek PTA, McCabe P, Pidcock E, et al. Mercury 4.0: from visualization to analysis, design and prediction. J Appl Crystallogr [Internet]. 2020;53:226–35. Available from: http://scripts.iucr.org/cgi-bin/paper?S1600576719014092.
Gowda BHJ, Nechipadappu SK, Shankar SJ, Chavali M, Paul K, Ahmed MG, et al. Pharmaceutical cocrystals of Efavirenz: towards the improvement of solubility, dissolution rate and stability. Mater Today Proc [Internet]. Elsevier Ltd; 2022;51:394–402. Available from: https://doi.org/10.1016/j.matpr.2021.05.535.
Wang L, Li S, Xu X, Xu X, Wang Q, Li D, et al. Drug-drug cocrystals of theophylline with quercetin. J Drug Deliv Sci Technol [Internet]. 2022;70:103228. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1773224722001381.
Chen X, Li D, Luo C, Wang J, Deng Z, Zhang H. Cocrystals of zileuton with enhanced physical stability. CrystEngComm [Internet]. 2018;20:990–1000. Available from: http://xlink.rsc.org/?DOI=C7CE02150J.
Belkafouf NEH, Triki Baara F, Altomare A, Rizzi R, Chouaih A, Djafri A, et al. Synthesis, PXRD structural determination, Hirshfeld surface analysis and DFT/TD-DFT investigation of 3N-ethyl-2N’-(2-ethylphenylimino) thiazolidin-4-one. J Mol Struct [Internet]. 2019;1189:8–20. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022286019304211.
Sousa EGR de, Carvalho EM de, San Gil RA da S, Santos TC dos, Borré LB, Santos-Filho OA, et al. Solution and solid state nuclear magnetic resonance spectroscopic characterization of efavirenz. J Pharm Sci [Internet]. 2016;105:2656–64. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022354915000076.
Skorupska E, Kaźmierski S, Potrzebowski MJ. Solid State NMR Characterization of ibuprofen:nicotinamide cocrystals and new idea for controlling release of drugs embedded into mesoporous silica particles. Mol Pharm. 2017;14:1800–10.
Nikam VJ, Patil SB. Pharmaceutical cocrystals of nebivolol hydrochloride with enhanced solubility. J Cryst Growth. 2020;534:125488. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022024820300117.
Shanthala HK, Jayaprakash HV, Radhakrishna M, Jaswanth Gowda BH, Paul K, Shankar SJ, et al. Enhancement of solubility and dissolution rate of acetylsalicylic acid via co-crystallization technique: a novel asa-valine cocrystal. Int J Appl Pharm. 2021;13:199–205.
Wardhana YW, Aisyah EN, Sopyan I, Rusdiana T. In vitro solubility and release profile correlation with pKa value of efavirenz polymorphs. Dissolution Technol. 2021;28:14–22. Available from: http://dissolutiontech.com/issues/202108/DT202108_Polymorphs.pdf.
Gowda BHJ, Ahmed MG, Shankar SJ, Paul K, Chandan RS, Sanjana A, et al. Preparation and characterization of efavirenz cocrystals: an endeavor to improve the physicochemical parameters. Mater Today Proc. Elsevier Ltd; 2022;57:878–86. Available from: https://doi.org/10.1016/j.matpr.2022.02.543.
Mannava MKC, Gunnam A, Lodagekar A, Shastri NR, Nangia AK, Solomon KA. Enhanced solubility, permeability, and tabletability of nicorandil by salt and cocrystal formation. CrystEngComm. 2021;23:227–37. Available from: http://xlink.rsc.org/?DOI=D0CE01316A.
Belenguer AM, Lampronti GI, De Mitri N, Driver M, Hunter CA, Sanders JKM. Understanding the influence of surface solvation and structure on polymorph stability: a combined mechanochemical and theoretical approach. J Am Chem Soc. 2018;140:17051–9. Available from: https://pubs.acs.org/doi/10.1021/jacs.8b08549.
Liu C, Liu Z, Chen Y, Chen Z, Chen H, Pui Y, et al. Oral bioavailability enhancement of β-lapachone, a poorly soluble fast crystallizer, by cocrystal, amorphous solid dispersion, and crystalline solid dispersion. Eur J Pharm Biopharm. 2018;124:73–81. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0939641117308974.
Fandaruff C, Rauber GS, Araya-Sibaja AM, Pereira RN, De Campos CEM, Rocha HVA, et al. Polymorphism of anti-HIV drug efavirenz: investigations on thermodynamic and dissolution properties. Cryst Growth Des. 2014;14:4968–75.
Booth BP, Simon WC. Analytical method validation. New Drug Dev Regul Paradig Clin Pharmacol Biopharm. 2016. p. 138–59.
Acknowledgements
Authors are thankful to Nirma University, Ahmedabad, for providing facilities for conducting research work, which is part of Doctor of Philosophy (PhD) of Dattatraya Yadav (18FTPHDP50), to be submitted to Nirma University, Ahmedabad, India. Authors are also thankful to Vaibhav Analytical Laboratory Ahmedabad for their kind support in analysis of the samples through gas chromatography. Authors are also thankful to Emcure R&D Center Gandhinagar, Ahmedabad, for their facilities for conducting research work.
Funding
The author of this article Dattatraya Yadav received a Chhatrapati Shahu Maharaj National Research fellowship (CSMNRF-2019) from the Chhatrapati Shahu Maharaj Research Training and Human Development Institute (SARTHI), Pune, Government of Maharashtra, India.
Author information
Authors and Affiliations
Contributions
DY: experimental work, methodology, validation, formal analysis, investigation, data curation, analysis and interpretation, writing—original draft, visualization; JS: methodology, formal analysis, analysis and interpretation, resources, writing—review and editing, supervision, project administration, revision and approval of the final version; KS: analysis, and interpretation of data for the work; AK: substantial contribution in an in vivo pharmacokinetic study; SP: substantial contribution in an in vivo pharmacokinetic study; all the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, D., Savjani, J., Savjani, K. et al. Pharmaceutical Co-crystal of Antiviral Agent Efavirenz with Nicotinamide for the Enhancement of Solubility, Physicochemical Stability, and Oral Bioavailability. AAPS PharmSciTech 24, 7 (2023). https://doi.org/10.1208/s12249-022-02467-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02467-7