Abstract
The freeze-drying process scale-up and transfer remain a complicated and non-uniform practice. We summarized inefficient and good practices in these papers and provided some practical advice. It was demonstrated that using the same process set points/times in laboratory and commercial scale dryers may lead to loss of product quality (collapse or vial breakage). The emerging modeling approach demonstrated practical advantages. However, the upfront generation of some input parameters (vial heat transfer coefficient, minimum controllable pressure, and maximum sublimation rate) is essential for model utilization. While the primary drying step can be transferred with a high degree of confidence (e.g., using modeling), and secondary drying is usually fairly straightforward, predicting potential changes in product behavior during freezing remains challenging.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The scale-up of the lyophilization process for commercial manufacturing has become an important topic since the early 2000s. In general, the lyophilization process transfer from the laboratory scale to the large scale requires a deep understanding of the formulation, process parameters, and equipment.
In principle, formulation defines a lyophilization process [1]. During process design and transfer, a particular concern is formulations with a very low maximum allowable product temperature during primary drying. If the maximum allowable product temperature is − 35°C or less, there is a high probability of problems during scale-up because of variability in equipment capability. A maximum allowable product temperature (during primary drying) of − 30°C or higher helps greatly in assuring scalability. Many formulations are very robust against damage caused by freeze-drying. That is, using more aggressive drying conditions results in more rapid drying. However, some commercial freeze-dryers (especially the old ones) may not support aggressive drying due to design limitations or lack of refrigeration capacity. Thus, knowing equipment capabilities would be helpful in the design of formulations and the associated lyophilization process.
Through experience in process scale-up, it is evident that each step of the freeze-drying process (freezing, primary, and secondary drying) may be influenced by the change in scale [1]. Thus, the goal of the scale-up process is to achieve similar product thermal history [2] between the laboratory and commercial processes during all lyophilization steps to provide the same product quality attributes and storage stability profile [3, 4].
During the freezing stage, the product is cooled to temperatures below the Tg’ to ensure conversion of water into ice and complete solidification of ice. The freezing protocol may include additional thermal treatment steps such as annealing to ensure complete crystallization of crystallizable excipients in the formulation and/or Ostwald ripening. The freezing step is followed by the primary drying phase, where ice is sublimed, leaving behind air pores (or voids). The porous structure of the cake determines the key attribute of the partially dried solids—the resistance to mass transfer. The freezing step directly affects/influences ice morphology (size and shape) and thereby the porosity. The temperature and duration of the annealing stage also affect the product’s porous structure. Thus, if the freezing profiles (freezing rate and degree of supercooling) in the lab and the commercial manufacturing are similar, the porous structure should be the same. However, in the manufacturing environment, due to particle-free conditions, a higher degree of supercooling [5] is observed that affects the morphology of ice crystals and, thereby, pore size leading to the differences in the product resistance (Rp). As manufacturing requires environments where only tiny particles are present (almost zero), the number of potential nucleation points in the product is much smaller than in laboratory environments. This leads to a higher degree of supercooling, which again affects the formation of (smaller) ice crystals. Smaller ice crystals result in smaller pore sizes and allow only lower vapor flow rates. The optimized annealing treatment can be applied to allow Ostwald ripening where larger crystals grow at the expense of smaller ones eliminating heterogeneity in ice size, thereby compensating for the differences with the lab-scale process.
Primary drying is usually the most time-consuming stage of the process and entails the most significant risk to product quality. Ice sublimation is carried out during this stage by applying heat and low pressure.
The unfrozen water is mainly removed from the product during the secondary drying stage by increasing the product temperature to promote diffusion through the glassy matrix to the solid’s surface, followed by evaporation. The secondary drying stage aims to reduce the final residual water content in the product to increase the glass transition temperature (Tg) and enhance the storage stability of the product. The shelf temperature setting during this stage is higher than in the primary drying stage, and the chamber pressure will be mostly the same or higher compared to primary drying, but the process usually takes only several hours.
At the laboratory scale, the recent advancements in process control made the lyophilization process development relatively easy. However, the scale-up to commercial scale is technically and logistically complicated and requires several modifications to the lab-scale process [6,7,8,9]. These modifications are the consequence of multiple factors described below.
There are important differences between freeze-drying in a pharmaceutical development laboratory and freeze-drying in a GMP manufacturing environment [10]. There may be significant differences in equipment design and capability; for example, the use of an internal condenser (condenser along the side walls or in the back of the chamber) versus a condenser connected to the chamber using a short duct, usually containing an isolation valve. Laboratory equipment commonly allows the freezing product to lower temperatures than production equipment and may allow ramping product temperature more rapidly. It is critical to understand equipment capability well enough to impose production equipment limitations on laboratory operations, thus avoiding unpleasant surprises during scale-up [11]. If possible, known differences should also be considered during lyophilization cycle development.
Laboratory freeze-drying operations generally occur in an environment that is not controlled in terms of airborne particulate matter, whereas the manufacturing environment is rigorously monitored and controlled [12]. Differences in airborne particulates can influence the freezing dynamics since particulate matter can act as a nucleation site for ice. There is a direct relationship between the degree of supercooling prior to ice nucleation and the effective drying rate [2]. Particularly, a high degree of supercooling is expected to result in smaller ice crystals, resulting in smaller channels in the porous bed of partially dried solids, a higher resistance of the solids to the flow of water vapor, and a slower sublimation rate. It should be noted that, while this explanation makes sense, we are aware of no published data on measured levels of supercooling in GMP freeze-dry operations. Regardless, it is important to be aware of differences in effective drying rate as a source of uncertainty in scale-up.
The difference in batch sizes should be noted as another source of uncertainty with respect to scale-up [13]. This may be important in dealing with challenges such as vial breakage and vial fogging [14, 15]. Multiple factors affect both issues, and neither one is thoroughly understood. Perhaps because of this, it has been generally difficult to reproduce the results of laboratory-scale investigations of either phenomenon, mainly when the incidence is low. It may not be until a much larger number of vials are involved that meaningful conclusions can be drawn about the magnitude of either problem.
Additional factors may include but are not limited to:
-
Parameters’ variations due to freeze-dryer geometry and materials
-
Design of lyophilization system (especially duct/main valve size and condenser size and cooling system)
-
Environmental conditions
-
Processing time (filling, loading, and unloading)
-
Measurement instrumentation technology differences in lab- and commercial-scale
-
Vacuum pump design (to a lesser extent)
-
Refrigeration capacity and condenser design differences between lab and full-scale dryers
Table I outlines the process parameters and product quality attributes that can be affected by the change of scale and potential challenges that may arise during the process.
Various solutions for the scale-up procedure have been developed in the past. The “as is” approach transfers the lyophilization cycles (either from laboratory to commercial dryer or between commercial dryers) without modification or with minimal adjustments. Such cycles may be suitable for robust products and processes and work well for dryers with similar characteristics. However, such transfer often results in a non-optimal but sufficient cycle for a particular product and could potentially result in catastrophic failures (see examples below).
Trial-and-error [16] is another strategy. It can involve a series of large-scale batches where one can make changes in process conditions that depend on the results of the previous batch. Generally, such an approach is not feasible from both time and cost perspectives. Indeed, the value of commercial biological drug product load is very high (reaching tens of millions of dollars), limiting the number of freeze-drying experiments on a large scale. Even though this approach is time-consuming and costly, understanding the factors affecting the scale-up and transfer can help further investigate the process.
One can consider the “robust cycle” approach as an alternative to the trial-and-error strategy, where the cycle on the pilot scale with adequate safety margins is designed [17]. The parameters for the pilot scale need to be carefully determined considering the differences with the production scale equipment.
In recent years, the modeling approach has been actively developed [8, 18, 19]. It is one of the most efficient approaches for scale-up and transfer because it is time- and cost-effective; it does not require expensive experiments. This approach is especially practical for the primary drying stage since this stage is the longest step in the lyophilization process. Note that this approach needs to be enhanced by continuous process monitoring of the lyophilization process (Pirani vs. capacitance manometer, tunable diode laser absorption spectroscopy (TDLAS), mass spectrometry, pressure rise test, etc.) to account for the variability of input parameters used in modeling.
Recognizing the importance of freeze-drying for manufacturing materials in bulk or dual-chamber containers, this paper will focus on drying products in vials as the most commonly used container closure. This work is intended to build upon previous “best practice” papers [20,21,22,23] developed by representatives of LyoHub member companies. This work aims to summarize best practices in scale-up and technology transfer based on past and current experiences and recommend best practices for the future. Both successful and unsuccessful practices from the industry are compiled, which are particularly useful when planning and designing the experiments. Part one will focus on challenges and unsuccessful practices, whereas the second will describe best practices and recommendations.
Challenges during Process Scale-up and Transfer
Freezing
Since freezing protocol directly impacts the degree of product resistance (Rp) during primary drying, and where Rp is one of the most important scale-up factors, it follows that controlling the cooling rate is paramount to the process. Ice crystal size is a key factor in the rate of the freeze-drying process [24]. A higher degree of supercooling, resulting in a faster freezing event, leads to smaller ice crystals [25], whereas a lower degree of supercooling will result in larger ice crystals. The consequence of either freezing event will impact, as mentioned in the introduction, the rate of primary drying, where smaller ice crystals will result in smaller pores in the cake during primary drying and higher resistance to mass transfer of water vapor, increasing primary drying time. Larger ice crystals will result in larger pores in the cake during primary drying and less resistance to mass transfer, reducing primary drying time. Furthermore, the selected cooling rate has additional consequences on the secondary drying step.
During freezing, the production class A/GMP manufacturing environment is also a major scale-up factor. Its impact on cake resistance is also considered in the following sections, in addition to the impact on the primary packaging itself and the resulting vial breakage described in the following section.
Differences between Two Production Dryers: Vial Breakage Observations
Vial breakage during freeze-drying is not a common but well-known event. It is typically associated with the crystallization of bulking agent such as mannitol [26] or some formulation excipients such as sodium chloride [27]). Vial breakage mostly occurs during the freezing step.
Freezing, the first step in the process, could be significantly influenced by both scale and specifics of a particular process, e.g., whether trays are used for loading of a freeze-dryer and the type of trays. In products with a crystalline bulking agent or other crystallizable solutes, an annealing step can be applied to ensure the complete crystallization of a solute. While annealing is common, it may introduce complications during cycle scale-up or transfer. In an example of a product transfer between two sites, a freeze-dried product, successfully manufactured in site A, was introduced to the second site (site B). When an engineering run for this product was performed at site B using the same set points as in site A (a freezing portion of the freeze-drying cycle presented in Fig. 1 as a red line), a significant (approx. 10%) vial breakage was observed. Vial breakage was not observed at site A. A main difference in the manufacturing setup was the use of trays with the bottom at the original site (site A), vs. bottomless trays at the receiving site (site B). It was considered that the less efficient shelf-to-vial heat transfer for trays with the bottom could reduce the product cooling rate compared with the bottomless tray set-up. It was further hypothesized that a faster product cooling rate at site B (with bottomless trays) could impact the crystallization behavior of a solute present (polyethylene glycol or PEG); the freezing behavior of the product was characterized by low-temperature X-ray diffraction, and crystallization of one of the components was observed (Fig. 1, middle). A stepwise cooling cycle (blue line, Fig. 1) was proposed to allow more time for crystallization at a relatively higher temperature. In this case, the mechanical stress on the vial could be reduced because of a reduced viscosity of the freeze-concentrated solution as well as a lower extent of the water-to-ice conversion; the latter mechanisms can be visualized with the help of a schematic ternary solid-liquid state diagram (Fig. 1, bottom). The stepwise cooling program was introduced for site B (Fig. 1, blue line), and vial breakage was avoided.
Top: Comparison between freezing profiles for the old cycle (red) and new cycle (blue). Middle: Synchrotron X-ray diffraction patterns at different temperatures, showing crystallization of PEG between −10 and −20°C. Bottom: Schematic solid-liquid-state diagram demonstrating that secondary solute + water crystallization at a lower temperature would produce more ice (the length of the horizontal arrows is proportional to the fraction of ice, based on the lever rule of phase diagrams). PEG + water crystallization at a lower temperature may result in higher mechanical stress: (1) higher viscosity = higher stress, (2) more ice formed = higher stress which facilitates crystallization at a higher temperature by using step-cooling
In another example, during a scale-up of a freeze-drying cycle developed in a 2.3-m2 lab-scale lyophilizer to a 25-m2 GMP lyophilizer for a small-molecule antibiotic formulation, a large amount of vial breakage of up to 18% was observed. A linear freezing ramp of 0.5°C/min from 5 to − 50°C was used in the lab-scale development cycle, followed by a hold for 5 h at − 50°C. Cracks towards the bottom of the vial up to the formulation fill height were noticed. It is important to note that although the lab-scale development cycle was run in a cleanroom in an ISO-7 environment, the conditions are significantly different from a class 100 GMP environment. Thus, comparing the nucleation temperatures indicated a higher degree of supercooling in the GMP lyo cycle. To solve this, an additional hold step at a temperature of −15°C for 3 h was introduced in the freezing step. This reduced the amount of vial breakage in the GMP lyo from 18.6 to 1.8%. This number was deemed acceptable by the drug product manufacturer, and the new recipe was implemented for future production runs after appropriate regulatory approvals.
Cake Resistance Differences
It is not unusual that commercial cycles are designed longer compared to laboratory cycles to account for multiple factors that impact the duration of different cycle steps, in particular primary drying. One of the factors is fewer particles in clean GMP facilities where lyophilized products are made.
Clean environments, typical for GMP conditions, result in a higher degree of supercooling and, consequently, in high specific surface area (SSA). An increase in SSA is associated with an increase in resistance of the dry product layer to the water vapor flow during sublimation, which is shown in Fig. 2.
The diagram on the left panel demonstrates the impact of particulate environments on the structure of ice post freezing and the apparent decrease of ice crystals size increasing specific surface area. The figure on the right panel demonstrates the relationship between specific surface area and resistance of the dry product layer: dotted lines—linear approximations (for demonstration purposes, the figure was redrawn using data presented by [2])
The relationship between mean cake resistance of laboratory and commercial materials can be roughly estimated using Eq. (1), where a is the slope in Fig. 2.
As follows from Fig. 2 (as a first approximation): for each 1°C increase in the degree of supercooling, one can assume an increase in resistance by about 5%. An increase in resistance results in a 10–30% increase in drying time for a 10° difference in supercooling (M. Pikal—verbal communication). Thus, using SSA measurements of both laboratory and commercial samples are very helpful in assessing scale-up factor during transferring cycles from laboratory to commercial scale. While SSA could be used to initially assess the scale-up factor and adjust process parameters or drying time during process design, PAT (Pirani vs. CM, TDLAS, PRT etc.) tools must be used to detect endpoints of drying (both primary and secondary drying steps), accounting for the variability of input parameters if modeling tools [18] are used for scaling up and transfer.
Differences in Heat Transfer Coefficient from the Heating Surfaces to Product between Lab-Scale and Commercial-Scale Dryers
It is known that most laboratory-scale dryers are fabricated with shelves with thicknesses around 12–13 mm. On the other hand, most recently made production freeze-dryer shelves are typically having a thickness of 15 mm, 18, or 21 mm. The reason could be a reinforcement needed at the commercial scale to stopper a large number of vials or to align with the current automatic loading and unloading systems (ALUS). The shelves of old freeze-dryers (up to 30 years old) could be even thicker due to the lack of technological advances at the time of manufacture. The thicker shelves have higher resistance to heat and, at the same temperature of heat transfer fluid at the shelf entrance, the shelf surface temperature of commercial dryers could be lower compared to laboratory dryers. This difference could be even higher at a higher thermal load (Eqs. 2 and 3 and Fig. 3)
The left panel demonstrates the difference in design between laboratory- and large-scale dryers. The right panel is the difference between inlet and shelf surface temperatures, shown as a function of the sublimation rate for laboratory and pilot dryers. Solid lines—linear approximations. (Reproduced from Tchessalov et al. [28])
Besides differences in design, old dryers could experience blockage of the channels within shelf reducing heat transfer from the heat transfer fluid to the shelf. This, therefore, will result in reduction of overall heat transfer coefficient of the shelf itself (see Equation 3).
Thus, in principle, to maintain the same normalized sublimation rate, shelf temperature in the commercial dryer should be adjusted from the laboratory cycle set point.
Figure 3 shows that the laboratory dryer has better heat transfer (smaller slope—right panel) than the pilot dryer. However, different observations were made by Rambahtla et al. [29] where commercial freeze-dryers had at least 2-fold better heat transfer as compared to old laboratory dryers. It is therefore essential to estimate the shelf heat transfer coefficient to guide the tech transfer process between scales or different commercial dryers.
How to determine this experimentally for each freeze-dryer (both at pilot and commercial scale) has been described in several previous publications [22, 30], and where it is recommended to perform an ice slab test in order to also determine the minimal achievable pressure as a function of sublimation rate, which is important risk mitigation when scaling up to different batch sizes (see the section below). The use of an ice slab, despite the logistical difficulties involved, is generally recommended over vials because a much higher depth of ice can be used instead of a smaller ice depth in vials. Ice in vials quickly loses contact with the vial wall, while ice in trays shrinks only slightly, thus providing more time for the test to be continued, resulting in more water vapor to be removed, i.e., more steady state points to gather through the experiment.
Differences in Vial Heat Transfer Coefficients between Laboratory and Commercial Dryers
A vial heat transfer coefficient, Kv, is defined by M. Pikal as the ratio of the area-normalized heat flow to the temperature difference between the heat source and heat sink [31]. For the vials directly sitting on the shelf of the dryer (most common case), he proposed an equation for Kv, as
In this paper, Pikal demonstrated that Kv is a function of pressure, radiation from the bottom and top of the shelf, and vials geometry. Thus, Kv needs to be measured for each vial of interest in the experiment. We assume that contact area, separation distance, and other factors play a role in the difference in Kv between vials [32].
The literature has reported that Kv of the same vial, measured gravimetrically, could drop by almost 27% when the product is transferred from the laboratory to the pilot dryer [33]. Therefore, during the transfer from dryer to dryer, one should pay attention to the location and diameter of sight glasses, differences in emissivity of shelves (impacts radiation contribution), or distance between shelves (impacts contribution from gas conductivity). It is desirable, at least for a limited set of vial nomenclature, to estimate Kv on a particular dryer (laboratory, pilot, or commercial), ensuring smooth tech transfer.
Measurements of shelf surface temperatures during Kv experiments are not a trivial task, especially for fully loaded dryers. Alternatively, one can use the heat transfer fluid temperature (replacing shelf surface temperature in Eq. 4). In this case, heat transfer through the shelf, described in the previous section, should be considered (see Eqs. 5 and 6).
One should be aware that if the commercial dryer is old and the shelf heat transfer coefficient is low, this could result in a notable difference in combined Kvs between laboratory and commercial dryers. The example in Fig. 4 shows about a 25% difference in Kvs (measured gravimetrically) between pilot and laboratory dryers. This example may be the worst-case scenario in terms of heat transfer for the old dryers (the estimated shelf heat transfer coefficient for this dryer is about 0.001548 Cal/s/cm2/K~65W/m2/K). New dryers are typically built with shelves with a greater heat transfer coefficient (300–400 W/m2/K). Therefore, the overall vial heat transfer coefficient may be comparable between modern laboratory and commercial dryers.
Shelf Temperature Uniformity
The uniformity of the shelf temperature across the shelf is a function of its fluid circulation path, narrowing of the fluid channel due to contamination, and unacceptable shelf curvature. Due to technological improvements in recent years, the shelf temperature uniformity in a clean, dry, and empty commercial lyophilizer is ± 2°C [20]. However, based on shelf design and construction, shelves between dryer types may have different distributions of “hot” and “cold” spots (Fig. 3) across their shelf area. Generally, it is safe to assume that dryer shelves cannot provide perfectly uniform temperatures across the entirety of the shelf [29].
It is fair to say that a source of non-uniformity in the vial heat transfer during primary drying is the circulation path of the fluid within the shelf. One should be aware that most commercially available lyophilizers employ a serpentine path for fluid flow wherein fluid enters the shelf through an inlet at one end and follows a winding path to the outlet located at the opposite end of the shelf. During the primary drying stage, the fluid enters at a high temperature and loses heat continuously as it flows through the shelf. The fluid leaving the shelf is at a lower temperature when compared to that entering, leading to a variation in the heat transfer characteristics to the vials along the fluid shelf path. The fluid flow rate within the shelf can significantly influence the heat transfer and affect the drying time and, thereby, the efficiency of the freeze-drying process. In addition to the flow rate provided by the pump, fluid properties such as viscosity also play a role in determining the velocity of the shelf fluid. The channel width and height control the flow rate of shelf fluid. The thickness of the shelf could further impact the heat transfer characteristics.
Computational fluid dynamics (CFD) modeling can be employed to analyze the impact of these factors on the shelf surface temperature the vials are exposed to and map the hot and cold spots within the lyophilizer. This study modeled fluid flow through a single shelf that loses heat at a constant flux of 500 W/m2 (corresponds to a constant sublimation flux of 0.8 kg/h/m2). The heat transfer characteristics of a 28 cm × 51 cm area shelf in a laboratory-scale lyophilizer were compared with a 50 cm × 50 cm shelf in a pilot-scale lyophilizer. In both cases, the fluid used in each lyophilizer enters the shelf inlet at 0.2 kg/s and −10°C. Figure 5 shows a comparison of the shelf surface temperature distributions. The average temperature on the surface of the pilot-scale lyophilizer is 0.26 °C lower than the laboratory-scale lyophilizer. This indicates that a higher mass flow rate or inlet temperature of shelf fluid is required at the pilot scale to maintain the same average shelf surface temperature as the laboratory scale.
Moreover, the temperature distribution on the shelf surfaces is uneven and representative of the serpentine path followed by the fluid. Hot and cold spots on the surface indicate intra-shelf variation of temperature. The difference in the flow path design between the two lyophilizers also results in shelf temperature differences. In the laboratory-scale lyophilizer, the fluid enters the shelf at the left bottom corner, as shown in Fig. 5, follows a serpentine path, and exits the shelf at the left top corner. In the pilot-scale lyophilizer, the fluid enters the shelf at the left bottom corner, follows the serpentine path, flows along a strain channel at the front of the shelf, and exits from the right bottom corner, as shown in the figure. Including radiation from the lyophilizer walls would further increase the non-uniformity in the surface temperature and vials that face the walls and door of the dryer. The final pass of colder shelf fluid along the strain channel at the front of the shelf in the pilot-scale lyophilizer could aid in counteracting the great edge effect from the lyophilizer door.
Consequently, the deviations in shelf uniformity between two lyophilizers generate a risk for unacceptable intra-batch variations in product temperature profiles, which can lead to unacceptable variability at the end of primary drying and unacceptable product quality attributes like residual moisture. During the validation run, placebo vials should be sampled for moisture mapping to understand the position-dependent temperature uniformity on shelves of the commercial lyophilizer. This factor should be considered important when a product is scaled-up from a newer pilot lyo to very old commercial lyophilizers that could have a lower rate of silicon fluid circulation inside their shelves. Overall, verification of the shelf temperature uniformity under full placebo load is strongly recommended during scale-up efforts to understand the inherent hot/cold spot distribution in the shelf heat map.
Edge Effect (Referred to as the Edge of Product Vial Array)
Atypical radiation heat transfer is well known and well documented in the literature [34]. It has to be accounted for during process scale-up and transfer since different dryers may have different edge effects. For example, if the chamber and condenser are separated, chamber walls and door temperatures usually are higher than product temperatures at the edge, and the edge effect could reach 40–50% (Fig. 6) of center vials. The negative edge effect (vials are facing lower than product temperatures) could, however, be observed when a thin plate only separates shelves with product and condenser within the same chamber or vials are facing condenser coils with no valve in between. Such temperature heterogeneity within the dryer would create different thermal histories for the product depending on location. It is therefore important to identify these “hot’ and “cold” locations and sample vials for analysis during the validation exercise. The sampling plan would therefore be different for dryers with an external condenser (center location and four corners on few shelves) and an internal condenser (corners, center, and location facing surface with the lowest temperature).
The left panel represents the edge effect (ratio of mass loss in edge vials to average mass loss in center vials) in the pilot dryer (manual loading, bottomless trays, 7.8 m2) and the edge effect in the commercial dryer (right panel, automatic loading, 23 m2). On the left panel, the edge effect is shown for the block of 4×5 vials, whereas in the right panel for the block 7×7. The shelves were heated to 0°C and held for approximately 2 h in both cases; wall temperature was not measured.
Impact of Equipment Design on Resistance to Mass Flow
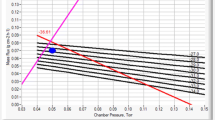
Freeze-dryer design can significantly impact the mass transfer during primary drying. The geometry of the freeze dryer, including the condenser placement with respect to the chamber and dimensions of the connecting duct, will strongly influence its minimum controllable chamber pressure and the maximum mass flow rate during sublimation. During the minimum controllable pressure test, a linear relationship is typically observed between the pressure and the corresponding maximum attainable sublimation rate (at lower chamber pressures, lower sublimation rates) (see Fig. 7, bottom panel). Additionally, the duct length to diameter ratio, especially its diameter, has a pronounced impact on the mass flow because high flow restrictions between chamber and condenser can generate a greater resistance to mass flux which, in turn, may result in a choked flow phenomenon. Therefore, the ratio of shelf area to the duct area and the ratio between duct length and its diameter are considered vital parameters in scale-up operations. For a successful process transfer, one should know the minimum controllable (achievable) pressure as a function of the sublimation rate for both sending and receiving dryers.
The left top panel represents minimum controllable pressure as a function of sublimation rate for laboratory dryers (green open squares, lab 1, 0.43 m2; green filled squares, lab 2, 0.74 m2), pilot dryer (blue filled triangles) and production dryer (purple filled circles). The right top panel shows the technical characteristics of dryers. The bottom left panel shows the relationship between achievable chamber pressure, condenser temperature, and sublimation rate for 41.5-m2 dryer. The bottom right panel shows the impact of the main valve size (600, 700, 800, and 900 mm) on the sublimation rate of a 21-m2 dryer
An example of freeze-dryer capabilities is shown in Fig. 7 (left and right top panels). Note that the relationship between minimum controllable pressure and sublimation rate can be presented differently where the equipment capability curve represents maximum sublimation rate at a given pressure (Fig. 7, bottom left panel). The size of the valve between chamber and condenser is also a major factor impacting freeze-dryer performance (Fig. 7, right bottom panel).
Another key factor that influences the mass flow during the sublimation step is the thickness and distribution of ice on the condenser coils. This factor is heavily influenced by the refrigeration system design of a freeze dryer. Ice accumulation on the condenser coils increases the ice layer’s temperature, which results in higher vapor pressure in the condenser, with a corresponding increase in the chamber pressure. Therefore, at higher chamber pressures, the minimum controllable pressure often has a nonlinear relationship with the maximum attainable sublimation rate) (see Fig. 7, bottom panel). The impact of the refrigeration system on additional factors important for the scale-up process is discussed further in the next subsection.
Differences in Equipment Cooling Rate and Effect of Refrigeration System
During the scale-up process, it is important to verify the variations in the cooling rate characteristics between lab and commercial lyophilizers. It is well known that the heat transfer capacity of silicon oil reduces as its temperature goes down due to the change in specific heat capacity, heat transfer coefficient, etc. [35]. The cooling rate of a freeze dryer is a matter of refrigeration system design, and an important parameter for comparing the lab and commercial FD performance can be the empty shelf cooling rate (or refrigeration capacity/shelf-area), which could be lower for commercial freeze-dryers. The loaded shelf cooling rate depends on the number of shelves loaded, vial type, and filling amount in the vials. These factors affect the shelf-cooling rate in big commercial FDs; therefore, the loaded shelf-cooling rate is typically not more than half of the empty shelf cooling rate. The significance of the refrigeration systems in the performance of freeze-dryers is shown in Table II.
It is noteworthy that for a compressor-based refrigeration system, the cooling capacity can be used by a shelf and/or ice condenser as per the process requirement, while in the case of LN2 refrigeration, dedicated systems are employed for shelf and ice condensers, respectively. Suppose the refrigeration system is designed with appropriate capacity (provided other equipment design parameters of Table II are not limiting). In that case, the desired cooling and specific sublimation rates can be obtained conveniently, irrespective of whether it is lab-/commercial-scale FD.
The impact of compressor design/LN2 system on refrigeration capacity with respect to evaporation temperature is shown in Fig. 8 (left panel). A more powerful refrigeration system can certainly improve shelf-cooling rates, but ice condenser performance would be improved only nominally. With an adequate size of refrigeration system already at disposal to obtain reasonable low temperatures, the extra benefit of additional compressors is not significant (see Fig. 8, right panel), while the energy consumption goes up.
Another factor is the uniformity of condenser coil temperatures, which is influenced by factors like variation in the inlet and outlet temperature of coils, flow structure, and the presence of non-condensable gasses. For example, an oversized refrigeration system/increase in pressure set points (meaning more nitrogen bleeding is occurring) could change ice deposition patterns on the ice condenser coils [36]. This could clog the water vapor path within the condenser (especially near the condenser inlet), creating more resistance and increasing minimum controllable pressure.
Impact of Freeze-Dryer Configuration (Condenser Position, Etc.)
The two most common types of condenser configuration seen in lyophilizers are external condensers where the product chamber and condenser are separated by a duct (also called ducted lyophilizers) and lyophilizers with an integral condenser where a hydraulically actuated plate separates the chamber and condenser. The choice between these two condenser types is primarily based on space considerations, but the uniformity of the drying rate should not be ignored during the scale-up process. The location of the integral condenser with respect to the chamber can influence the wall temperature and pressure uniformity with a downstream influence on the heat transfer to product vials. For example, a side integral condenser can have a solid potential to cool down the divider wall due to exposure to the condenser coils, with severe implications for any scale-up effort. Overall, the uniformity of the wall temperature in the product chamber is highly desirable for any scale-up effort, and the difference in the uniformity of the heat transfer to product vials between two lyophilizers should be evaluated in any scale-up effort. As an example, for 40 m2 integral lyophilizer (i.e., condenser on one side of the chamber constructed in a single vessel and separated by a wall), the difference in wall temperatures could reach up to 20°C, creating different edge effects for the product on the opposite side of shelves.
Differences in Process Control and Design (Temperature and Pressure Control, Capacitance Manometer Vs. Pirani, Condenser Capacity, Etc.)
The accuracy of pressure and shelf temperature control could vary with the scale. Typically, fluctuation of process parameters from the set point increase with an increase in scale (an example is shown in Fig. 9). Also, sometimes successful laboratory cycle recipes may not be implemented at a larger scale due to more simplified process control at a clinical or commercial scale. For example, a laboratory cycle shown in Fig. 9 was changed from 3-step pressure to a single-step pressure cycle due to the limitation of pressure control at the clinical dryer.
Comparison between cycle parameters used for the same product on laboratory (left panel) and pilot dryers (right panel). Note larger fluctuations in shelf temperature and pressure in pilot dryer (±3.8°C/±4.6 mT) as compared to the laboratory dryer (±1.3°C/±0.7 mT). Fluctuations were calculated as three standard deviations from the average of process parameter values during primary drying hold time
Instrumental deviations in shelf temperature measurement can also adversely affect the scale-up process. For example, if the shelf inlet probe is positioned at the chamber entrance, it is ideal for minimizing the lag time from the control point, the refrigeration solenoid valve. The lag time is the time it takes for a droplet of fluid to go from the evaporator heat exchanger to the shelf inlet temperature probe. The shelf fluid temperature deviation may become a source of variability when a lyo-process is scaled up from a more modern lyophilizer that can sustain a heat exchange fluid velocity of 5–10 ft per s as compared to some legacy lyophilizers that may show a greater temperature lag due to a lower sustained fluid velocity of 2–4 ft per s. Additionally, some older lyophilizers have shelf temperature measurement probes positioned at the shelf outlet, which can be 2 to 5°C warmer than shelf fluid inlet temperature due to the passage of the heat exchange fluid below product vials positioned on the shelf. In summary, it is important to quantify the variations in shelf temperature uniformity between laboratory and GMP lyo to minimize the chance of scale-up failure.
In addition, it could be a difference between accuracy of temperature measurement between laboratory and commercial freeze-dryers due to the different types of sensors used. The thermocouples are commonly used in laboratory dryers for the temperature measurement of heat transfer fluid and condenser temperatures. While for the most commercial dryers more accurate, easy calibrated and reproducible RTDs are normally utilized. The pressure sensors at commercial scale are exposed to the multiple steam sterilization cycles and, therefore, require more frequent calibration. Note that, in some cases, Pirani used at the commercial scale for pressure control which require adjustment of pressure set points from laboratory cycles accounting for the difference in reading between CM and Pirani during primary drying [20].
Impact of Container Treatment on Product Appearance during Transfer: Vial Fogging
Vial fogging during lyophilization is not an uncommon problem that could potentially result in the rejection of commercial products even though product quality may remain within specification. In the first example, after the freeze-drying process, a haze of dried powder (film) was visible inside the drug product vial (Fig. 10, left panel), making it barely acceptable for commercial distribution from a cosmetic point of view which could result into a market complaint. All the lab-scale robustness studies were conducted on the unprocessed glass; hence, no fogging was observed on the lab scale in this case. However, authors also observed fogging with different material/vial combinations at laboratory scale as well as variability in vial fogging with commercial scale (Fig. 10, right panel). Some authors also observed that fogging primarily depends on the pre-treatment process of the glass vials, especially the temperature and duration they spend in a heat tunnel. However, they only see this issue with some formulations which contained surfactants and formulated antibodies or conjugates.
Formulation composition (type and concentration of surfactant, including a control group with no surfactant), glass vial processing (washing/depyrogenation) and composition (non-siliconized vs. siliconized inner vial surface), and thermal history of the filled vials (holding for extended times at −20 to −30°C) were investigated to understand the impact on fogging. Fogging was reported [14] even in the formulation group containing no surfactant. One way to minimize or eliminate fogging was to use a glass vial with a siliconized inner surface. In another study [37], annealing positively impacted fogging reduction. However, this observation was not confirmed with different products by some authors of this paper.
Some authors of this paper report this phenomenon as having occurred at a commercial scale, with extensive fogging and a capillary effect on the glass vial wall, even when the same vial primary packaging was used at the pilot scale, with the same freeze-drying cycle and product, showed no such effect. This further demonstrates a clear scale-up issue and poor ability to predict fogging at a commercial scale. Therefore, fogging remains a very complex, hardly predictable phenomenon that usually worsens at the commercial scale but may not be a necessary common event.
Impact of Vial Siliconization on Cake Appearance
While siliconization could significantly decrease vial fogging and, in some cases, demonstrate no impact on product quality [14, 38], it could create a problem by itself. Figure 11 shows challenges with siliconization—formation of “half-moon” cakes for very low fill volume products. The issue can be resolved with a reduced speed of the filling line and the use of vials with smaller diameters, but it could take some time to fix this problem at the commercial scale.
The paper’s authors also found that using the vial with a hydrophobic inner surface has resulted in significant cake shrinkage, giving the impression of partial collapse.
Effect of Product Load on the Freeze-Drying Process
It is not very uncommon in the pharmaceutical/biopharmaceutical industry to see variations in the dryer load (batch sizes) during commercial manufacturing, going from full capacity to partial loads and vice versa for various reasons [39]. One must be aware that variations in product load in the freeze-dryer would influence the thermal history of the product in different ways, which are briefly discussed below.
If the process is a high sublimation rate process where the shelf temperature set point for primary drying is above 0°C, the process may run fine at partial load. However, whenever it becomes necessary to run at 100% of full load, dryer mass and heat transfer limitations may result in dryer overload leading to loss of pressure control, and the product would overheat and suffer the loss of quality. It is also possible in the case of a high sublimation rate process that a partial load, particularly with less than 100% load on each shelf, the heat needed during primary drying will be less due to less product load resulting in the shelf surface temperature to be somewhat higher than with full load. Let’s consider a case in which the shelf temperature is controlled with regard to silicone oil outlet temperature. Since the FD is partially loaded, the silicone oil will flow through the empty shelves with no heat loss. This will lead to an overall higher silicone oil outlet temperature. Thus, the controller would reduce the inlet silicone oil temperature, leading to a lower sublimation rate.
Another way the product load can influence the thermal product history is when the load on any shelf decreases, a greater fraction of the vials become either edge vials or vials somewhat influenced by the greater heat transfer to edge vials via radiation [34] resulting in a greater percentage of vials experiencing higher product temperature. Lastly, it is also possible that as the fractional load decreases, at some point when the number of vials is simply too small to maintain the molar flux of water vapor higher than the flux of nitrogen, the gas composition in the chamber will shift from water vapor to nitrogen thereby decreasing the heat transfer coefficient governing heat transfer from shelf to product (needs to be confirmed experimentally).
Patel et al. [39] performed a systematic study of the effects of variation in product load on freeze-drying behavior in the laboratory, pilot/clinical-scale freeze-dryers. They concluded that under partial load conditions, the average shelf surface temperature increases, and the fraction of edge vials increases, leading to an increase in radiation effect. However, they observed minimal change in the composition of vapor. The increase in radiation effect can directly influence the heat transfer coefficient, resulting in increased product temperature and decreased primary drying time, requiring optimization of the process to have an identical product temperature history. These findings are critical, especially for commercial scale freeze dryers with high thermal load cycles.
Performing such studies during the process design stage will help to quickly and accurately judge the impact of running at less than the full load on the freeze-drying process, and more importantly, it will help better design the batch sizes (Min and Max) for validation. Failing to characterize the effect of product load on the process performance would result in the continuation of high heterogeneity in product quality attributes and continuation of present procedures for making such decisions, involving trial and error and guesswork.
As for the arrangement of vials in production freezer-dryers for partial loads, the recommendation is to fill as many shelves as possible and leave the remaining unfilled shelves extended and empty during the cycle. The empty shelves would collapse during stoppering. For partial shelves, the product vials are placed in the center of the array, and the remainder of the shelf is filled with empty vials to ensure that the vials do not move during the cycle and uniform weight distribution for smooth stoppering.
Impact of Shelf Roughness during Scale-Up
In this example, a drug product manufacturer was experiencing unacceptable vial dislocations during the stoppering step. This was due to the sticking of stoppers on the underside of the shelf, causing the vials. However, this issue was not observed during cycle development in a smaller scale R&D lyo.
A preliminary analysis showed that the root cause of this discrepancy was due to a difference in shelf surface roughness between the R&D Lyo (roughness of 3.3 Ra (μ)) versus a GMP Lyo (roughness values typically between 0.4 and 0.6 Ra (μ)). A stoppering study with identical pressure, shelf temperature, stoppering force, and chamber pressure, but different shelf roughness in the same lyo revealed significant differences. Zero incidents of stopper sticking were seen on the Lyo shelf with 3.3 Ra (μ) roughness, while 13.7% stopper sticking was observed on the Lyo shelf with 0.4–0.6 Ra (μ) roughness.
It is important to note that while this issue is not generated by process scale-up difference between lab and GMP lyophilizer but rather design differences, it is one of the factors that should be kept in mind during scale-up.
Unknown Factors
In many cases, the impact of scale during lyophilization cycle transfer may be somewhat predicted based on experience and process knowledge available to date. However, there are examples of unexpected observations documented during process scale up, with one example provided in Fig. 12. In this example, higher turbidity of the reconstituted solution was found in a product produced on a pilot dryer, while the same product manufactured with a laboratory freeze-dryer formed a transparent solution after reconstitution. The turbidity was confirmed to be due to air bubbles [40]. The difference in the degree of supercooling (8.1±0.6°C on the lab scale vs −14.2±2.8°C in the pilot manufacture) at the pilot scale was considered but not proven yet to be the main contributor.
The summary of unusual, non-predictable observations at scale is shown in Table III.
Conclusions/Summary
Many factors impact product quality during the lyophilization process scale-up and transfer. Differences in scale and environmental conditions dictate differences in heat and mass transfer between laboratory and commercial scales. Among the factors evaluated were equipment differences (minimum controllable pressure, vial heat transfer coefficients, edge effect, limitations of refrigeration systems, etc.) and process attributes (degree of supercooling, vial breakage, etc.). Considering all these factors while scaling up from a lab scale to a production freeze-dryer is essential since they all impact the final product quality. This paper summarizes factors influencing the tech transfer and scale-up supported by case studies. Following this, in a forthcoming paper, members of the LyoHUB and BPOG will share past and current practices and provide some guidance and best practice recommendations for a successful lyophilization process scale-up and tech transfer.
Abbreviations
- A sh :
-
Shelf surface area, m2
- A out :
-
Cross-sectional area of the vial calculated from the vial outer diameter, cm2
- a :
-
Slope in graph Rp=f(SSA), Torr*h
- ΔH S :
-
Heat of sublimation, Cal/g
- K s :
-
Shelf heat transfer coefficient, Cal/s/cm2/K
- K v :
-
Vial heat transfer coefficient, Cal/s/cm2/K
- K v,s :
-
Vial/shelf combination heat transfer coefficient, Cal/s/cm2/K
- k effective :
-
Effective shelf heat conductivity coefficient over the average distance from heat transfer fluid to shelf surface, Cal/K/s/cm
- L effective :
-
Effective thickness of shelf (average shelf thickness assumed as a distance from heat transfer fluid to the shelf surface), cm
- dm/dt :
-
Sublimation rate, kg/h
- (R p )com :
-
Normalized cake resistance of material after lyophilization on the commercial freeze-dryer (Torr*cm2*h/g);
- (R p )lab :
-
Normalized cake resistance of material after lyophilization on the laboratory freeze-dryer (Torr*cm2*h/g);
- SSA com :
-
Specific surface area of samples from commercial freeze-dryer, m2/g
- SSA lab :
-
Specific surface area of samples from laboratory freeze-dryer, m2/g
- T bottom :
-
Temperature of the product at the bottom center of the vial, °C
- T Shelfinlet :
-
Inlet temperature of heat transfer fluid, °C
- T shelfsurface :
-
Average temperature of shelf surface, °C
- T Sh.S :
-
Temperature of shelf surface, °C
- ΔT error :
-
Difference between temperature of heat transfer fluid, measured by sensor and true inlet temperature, °C
- α HTF :
-
Average heat transfer coefficient from heat transfer fluid to internal surface of shelf channels, Cal/s/cm2/K
References
Bhatnagar B, Tchessalov S, Lewis L, Johnson R. Freeze-drying of biologics. Encyclopedia of Pharmaceutical Science and Technology, 4th edition. Publisher Taylor & Francis; p. 1673–1722
Rambhatla S, Ramot R, Bhugra C, Pikal MJ. Heat and mass transfer scale-up issues during freeze drying: II. Control and characterization of the degree of supercooling. AAPS PharmSciTech. 2004;5(4):54–62.
Jennings TA. Transferring the lyophilization process from one freeze-dryer to another. Am Pharm Rev. 2002;5(1):34–42.
Jameel F, Paranandi M. Critical considerations in the transfer and validation of a lyophilization process. Am Pharm Rev. 2006;9(3):53–5.
Pikal MJ. Freeze drying. In: Swarbrick J, ed. Encyclopedia of Pharmaceutical Technology, 3rd edn. New York, NY: Informa Healthcare USA, Inc, 2006: 1807–33.
Tchessalov S, Dixon D, Warne N. Principles of lyophilization cycle scale-up. Am Pharm Rev. 2007;10(3):88.
Trappler E. Scale-up strategy for a lyophilization process. Am. Pharm. Rev. 2001;4:55–6,58,60. Russell Publishing LLC.
Tchessalov S. Principals of lyophilization scale-up. CPPR (Center for Pharmaceutical Processing Research) Freeze Drying of Pharmaceuticals and Biologicals Conference,Garmisch-Partenkirchen. 2006.
Kamat M, Varshney D. Lyophilization Process Technology Transfer Towards Product Launch, in Lyophilized Biologics and Vaccines: Modality-Based Approaches, D. Varshney and M. Singh, Editors. 2015, Springer New York: New York, NY p 381-397.
Gamiz AGR, Dewulf J, De Soete W, Heirman B, Dahlin P, Jurisch C, et al. Freeze drying in the biopharmaceutical industry: an environmental sustainability assessment. Food Bioprod Process. 2019;117:213–23.
Kuu WY, Hardwick LM, Akers MJ. Correlation of laboratory and production freeze drying cycles. Int J Pharm. 2005;302(1-2):56–67.
Nail SL, Gatlin LA. Freeze-drying: principles and practice. In pharmaceutical dosage forms-parenteral medications (pp. 367-396). CRC Press. 2016.
Ohori R, Akita T, Yamashita C. Scale-up/tech transfer issues of the lyophilization cycle for biopharmaceuticals and recently emerging technologies and approaches. Dry Technol. 2021. https://doi.org/10.1080/07373937.2021.2015372.
Abdul-Fattah AM, Oeschger R, Roehl H, Dauphin IB, Worgull M, Kallmeyer G, et al. Investigating factors leading to fogging of glass vials in lyophilized drug products. Eur J Pharm Biopharm. 2013;85(2):314–26.
Langer C, Mahler H-C, Koulov A, Marti N, Grigore C, Matter A, Chalus P, Singh S, Lemazurier T, Joerg S, Mathaes R. Method to predict glass vial fogging in lyophilized drug products. J Pharm Sci. 2020 Jan;109(1):323–30.
Tsinontides SC, Rajniak P, Pham D, Hunke WA, Placek J, Reynolds SD. Freeze drying—principles and practice for successful scale-up to manufacturing. Int J Pharm. 2004;280(1-2):1–16.
Sane SU, Hsu CC. Considerations for successful Lyophilization process scale-up, technology transfer, and routine production. Form Process Dev Strate Manuf Biopharm. 2010:797–826.
Zhu T, Moussa EM, Witting M, Zhou D, Sinha K, Hirth M, et al. Predictive models of lyophilization process for development, scale-up/tech transfer and manufacturing. Eur J Pharm Biopharm. 2018;128:363–78.
Shivkumar G, Kazarin PS, Strongrich AD, Alexeenko AA. LyoPRONTO: an open-source Lyophilization process optimization tool. AAPS PharmSciTech. 2019;20(8):1–17.
Nail S, Tchessalov S, Shalaev E, Ganguly A, Renzi E, Dimarco F, Wegiel L, et al. Recommended best practices for process monitoring instrumentation in pharmaceutical freeze drying—2017. Aaps Pharmscitech. 2017;18(7):2379–93.
Ganguly A, Hardwick L, Tchessalov S, Nail S, Dixon D, Kanka F, Guidinas A, Thompson T, Reiter C, Yusoff Z, Tharp T, Azzarella J, Sharma P, Kazarin P, Alexeenko A, Pikal M. Recommended best practices in freeze dryer equipment performance qualification: 2021, AAPS Pharmscitech. 2022 Paper is in the review process.
Jameel F, Alexeenko A, Bhambhani A, Sacha G, Zhu T, Tchessalov S, Kumar L, Sharma P, Moussa E, Iyer L, Fang R, Srinivasan J, Tharp T, Azzarella J, Kazarin P, Jalal M. Recommended Best Practices for Lyophilization Validation—2021 Part I: Process Design and Modeling. AAPS PharmSciTech. 2021;22:221.
Jameel F, Alexeenko A, Bhambhani A, Sacha G, Zhu T, Tchessalov S, Kumar L, Sharma P, Moussa E, Iyer L, Fang R, Srinivasan J, Tharp T, Azzarella J, Kazarin P, Jalal M. Recommended Best Practices for Lyophilization Validation 2021 Part II: Process Qualification and Continued Process Verification. AAPS PharmSciTech. 2021;22:266.
Bogdanova E, Fureby AM, Kocherbitov V. Influence of Cooling rate on ice crystallization and melting in sucrose-water system. J Pharm Sci. 2022:1–8. https://doi.org/10.1016/j.xphs.2022.01.027.
Lu X, Pikal MJ. Freeze-drying of mannitol–trehalose–sodium chloride-based formulations: the impact of annealing on dry layer resistance to mass transfer and cake structure. Pharm Dev Technol. 2004;9(1):85–95.
Williams NA, Lee Y, Poll GP, Jennings TA. The effects of cooling rate on solid phase transitions and associated vial breakage occurring in frozen mannitol solutions. PDA J Pharm Sci Technol. 1986;40(4):135–41.
Milton N, Gopalrathnam G, Craig GD, Mishra DS, Roy ML, Yu L. Vial breakage during freeze-drying: crystallization of sodium chloride in sodium chloride-sucrose frozen aqueous solutions. J Pharm Sci. 2007;96(7):1848–53.
Tchessalov S, Luthra S, Thompson TN, Graham L. Science of scale for freeze-drying. In: Parenteral Medications, 4th edition. CRC Press, publisher Taylor & Francis; 2019. p. 785–808.
Rambhatla S, Tchessalov S, Pikal MJ. Heat and mass transfer scale-up issues during freeze-drying, III: control and characterization of dryer differences via operational qualification tests. AAPS PharmSciTech. 2006;7(2):E61–70.
Kshirsagar V, Tchessalov S, Kanka F, Hiebert D, Alexeenko A. Determining maximum sublimation rate for a production lyophilizer: computational modeling and comparison with ice slab tests. J Pharm Sci. 2019;108(1):382–90.
Pikal MJ, Roy ML, Shah S. Mass and heat transfer in vial freeze-drying of pharmaceuticals: role of the vial. J Pharm Sci. 1984;73(9):1224–37.
Kuu WY, Nail SL, Sacha G. Rapid determination of vial heat transfer parameters using tunable diode laser absorption spectroscopy (TDLAS) in response to step-changes in pressure set-point during freeze-drying. J Pharm Sci. 2009;98(3):1136–54.
Kramer T, Kremer DM, Pikal MJ, Petre WJ, Shalaev EY, Gatlin LA. A procedure to optimize scale-up for the primary drying phase of lyophilization. J Pharm Sci. 2009;98(1):307–18.
Rambhatla S, Pikal MJ. Heat and mass transfer scale-up issues during freeze-drying, I: atypical radiation and the edge vial effect. AAPS PharmSciTech. 2003;4(2):22–31.
Zhai, et al. Measurement and Analysis of Silicone Oil Characteristics and Viscosity-Temperature Index. IOP Conf Series: Earth Environ Sci. 2019;330:3.
Kobayashi M. Important problems on industrial freeze-dryers: mainly on the vapor removing performance and its control. Science et Technique du Froid (France). 1985.
Huang M, Childs E, Roffi K, Karim F, Juneau J, Bhatnagar B, et al. Investigation of fogging behavior in a lyophilized drug product. J Pharm Sci. 2019;108(3):1101–9.
Patel SM, Nail SL, Pikal MJ, Geidobler R, Winter G, Hawe A, Davagnino J, Gupta SR. Lyophilized drug product cake appearance: what is acceptable? J Pharm Sci. 2017;106(7):1706–21.
Patel SM, Jameel F, Pikal MJ. The effect of dryer load on freeze drying process design. J Pharm Sci. 2010;99(10):4363–79.
Shalaev E, Hill JJ. Interfacial Stress and Proteins Prepared in the Solid State. In: Protein Instability at Interfaces During Drug Product Development. J Li, ME Krause, R Tu (Eds) Springer; 2021. pp. 271-287
Funding
Serguei Tchessalov: N/A
Evgenyi Shalaev: N/A
Bakul Bhatnagar: N/A
Steven Nail: N/A
Alina Alexeenko: Paid employee of LyoHUB, Purdue University, at the time of the project/paper drafting process.
Feroz Jameel: N/A
Jayasree Srinivasan: N/A
Michael Dekner: N/A
Ekneet Sahni: N/A
Stefan Schneid: N/A
Petr Kazarin: Paid employee of LyoHUB, Purdue University, at the time of the project/paper drafting process.
Orla McGarvey: N/A
Bert Van Meervenne: N/A
Vaibhav Kshirsagar: N/A
Paritosh Pande: N/A
Jens Philipp: N/A
Greg Sacha: N/A
Ke Wu: N/A
Joseph Azzarella: N/A
Gayathri Shivkumar: N/A
Shreyas Bhatt: N/A
Author information
Authors and Affiliations
Contributions
Serguei Tchessalov: Paper leadership and substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Evgenyi Shalaev: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Bakul Bhatnagar: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Steven Nail: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Alina Alexeenko: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Feroz Jameel: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jayasree Srinivasan: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Michael Dekner: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ekneet Sahni: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Stefan Schneid: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Petr Kazarin: Responsible for consolidating the information contributed by all the authors and compiling it into a cohesive single document. He contributed to the introduction section, was responsible for editing the contents of the contributions and discussing with authors regarding the information they provided for its clarity.
Orla McGarvey: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Bert Van Meervenne: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Vaibhav Kshirsagar: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Paritosh Pande: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jens Philipp: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Greg Sacha: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ke Wu: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Joseph Azzarella Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and Drafting the work or revising it critically for important intellectual content; and Final approval of the version to be published; and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Gayathri Shivkumar: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Shreyas Bhatt: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tchessalov, S., Shalaev, E., Bhatnagar, B. et al. Best Practices and Guidelines (2022) for Scale-Up and Tech Transfer in Freeze-Drying Based on Case Studies. Part 1: Challenges during Scale Up and Transfer. AAPS PharmSciTech 24, 11 (2023). https://doi.org/10.1208/s12249-022-02463-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02463-x