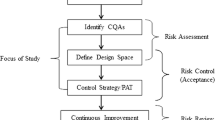
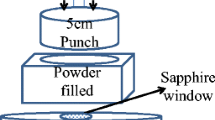
Abstract
Blending is a critical intermediate unit operation for all solid oral formulations. For blend uniformity testing, API content in the blend must be quantified precisely. A detailed study was conducted to demonstrate the suitability of inline NIR (near-infrared) spectroscopy for blend uniformity testing of two solid oral formulations: existing direct compression (DC) product with a multistep blending process and granulation-based product with API granules. Both qualitative and quantitative methods were developed at a laboratory scale using statistical moving block standard deviation (MBSD) and multivariate data analysis such as principal component analysis (PCA) and partial least squares (PLS) regression. The qualitative MBSD method demonstrated that there was no need for multiple steps for the existing DC product. Hence, a simplified single-step process was developed for blending. Quantitative PLS models for blending processes of both the products were developed, validated, and successfully implemented at a commercial scale for the real-time release of blends. Results obtained from the validated model were in good agreement with the current method of sampling and chromatography.
Graphical abstract









Similar content being viewed by others
References
ICH harmonized tripartite guideline, Pharmaceutical Development Q8 (R2), 2009. https://database.ich.org/sites/default/files/Q8%28R2%29Guideline.pdf.
US Food and Drug Administration, Guidance for industry, PAT-A framework for innovative pharmaceutical development, manufacturing and quality assurance, 2004. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070305.pdf.
Zhong L, Gao L, Li L, Zang H. Trends-process analytical technology in solid oral dosage manufacturing. Eur J Pharm Biopharm. 2020;153:187–99. https://doi.org/10.1016/j.ejpb.2013.01.016.
Laske S, Paudel A, Scheibelhofer O. A review of PAT strategies in secondary solid oral dosage manufacturing of small molecules. J Pharm Sci. 2017;106(3):667–712. https://doi.org/10.1016/j.xphs.2016.11.011.
Wahl P, Fruhmann G, Sacher S, Straka G, Sowinski S, Khinast J. PAT for tableting: inline monitoring of API and excipients via NIR spectroscopy. Eur J Pharm Biopharm. 2014;87(2):271–8. https://doi.org/10.1016/j.ejpb.2014.03.021.
Fonteyne M, Vercruysse J, De Leersnyder F, Van Snick B, Vervaet C, Remon J, De Beer T. Process analytical technology for continuous manufacturing of solid-dosage forms. Trends Anal Chem. 2015;67:159–66. https://doi.org/10.1016/j.trac.2015.01.011.
Riolo D, Piazza A, Cottini C, Serafini M, Lutero E, Cuoghi E, et al. Raman spectroscopy as a PAT for pharmaceutical blending: advantages and disadvantages. J Pharm Biomed Anal. 2018;149:329–34. https://doi.org/10.1016/j.jpba.2017.11.030.
Rantanen J. Process analytical applications of Raman spectroscopy. J Pharm Pharmacol. 2007;59:171–7. https://doi.org/10.1211/jpp.59.2.0004.
Beer T, Burggraeve A, Fonteyne M, Saerens L, Remon JP, Vervaet C. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. Int J Pharm. 2011;417:32–47. https://doi.org/10.1016/j.ijpharm.2010.12.012.
Taday P. Applications of terahertz spectroscopy to pharmaceutical sciences. Philos Trans A Math Phys Eng Sci. 1815;2004(362):351–63. https://doi.org/10.1098/rsta.2003.1321.
Ajito K. Terahertz spectroscopy for pharmaceutical and biomedical applications. IEEE Transactions on Tetrahertz Sci and Tech. 2015;5(6):1140.
Guay JM, Lapointe-Garant PP, Gosselin R, Simard JS, Abatzoglou N. Development of a multivariate light-induced fluorescence (LIF) PAT tool for in-line quantitative analysis of pharmaceutical granules in a V-blender. Eur J Pharm Biopharm. 2014;86:524–31. https://doi.org/10.1016/j.ejpb.2013.12.013.
Lin H, Zhang Z, Markl D, Zeitler J, Shen Y. A review of the applications of OCT for analysing pharmaceutical film coatings. Appl Sci. 2018;8:2700. https://doi.org/10.3390/app8122700.
Barton F. Theory and principles of near infrared spectroscopy. Spectrosc Eur. 2002;14(1):12–8.
Roggo Y, Chalus P, Maurer L, Lema-Martinez C, Edmond A, Jent N. A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies. J Pharm Biomed Anal. 2007;44(3):683–700. https://doi.org/10.1016/j.jpba.2007.03.023.
Yu L, Amidon G, Khan M, Hoag S, Polli J, Raju G, Woodcock J. Understanding pharmaceutical quality by design. The AAPS Journal. 2014; 16(4):771–783. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4070262/
Muzzio F, Robinson P, Wightman C, Dean B. Sampling practices in powder blending. Int J Pharm. 1997;155:153–78. https://doi.org/10.1016/S0378-5173(97)04865-5.
Berman J. The compliance and science of blend uniformity analysis. PDA J Pharm Sci Technol. 2001;55(4):209–22.
Boehm G, Clark J, Dietrick J, Foust L, Garcia T, Gavini M, Gelber L, Goeffroy JM, Jimenez P, Mergen G, Muzzio F, Planchard J, Prescott J, Timmermans J, Takiar N. The use of stratified sampling of blend and dosage units to demonstrate adequacy of mix for powder blends. PDA J Pharm Sci Tech. 2003;57(2):59–74.
Muzzio F, Goodridge C, Alexander A, Arratia P, Yang H, Sudah O, Mergen G. Sampling and characterization of pharmaceutical powders and granular blends. Int J Pharm. 2003;250:51–64. https://doi.org/10.1016/S0378-5173(02)00481-7.
US Food and Drug Administration, Guidance for industry, development and submission of near infrared analytical procedures, 2021.https://www.fda.gov/media/91343/download
Abatzoglou N, Lapointe-Garant P, Simard J. Real-time NIR monitoring of pharmaceutical blending processes with multivariate quantitative models. Eur Pharmacol Rev. 2009;5:57–67.
Berntsson O, Danielsson L, Lagerholm B, Folestad S. Quantitative in-line monitoring of powder blending by near infrared reflection spectroscopy. Powder Technol. 2002;123:185–93. https://doi.org/10.1016/S0032-5910(01)00456-9.
Igne B, Juan A, Jaumot J, Lallemand J, Preys S, Drennen J, Anderson CA. Modeling strategies for pharmaceutical blend monitoring and end-point determination by near-infrared spectroscopy. Int J Pharm. 2014;473:219–31. https://doi.org/10.1016/j.ijpharm.2014.06.061.
Rajalahti T, Kvalheim O. Multivariate data analysis in pharmaceutics: a tutorial review. Int J Pharm. 2011;417(1–2):280–90. https://doi.org/10.1016/j.ijpharm.2011.02.019.
Ferreira A, Tobyn M. Multivariate analysis in the pharmaceutical industry: enabling process understanding and improvement in the PAT and QbD era. Pharm Dev Technol. 2015;20(5):513–27. https://doi.org/10.3109/10837450.2014.898656.
Sekulic SS, Wakeman J, Doherty P, Hailey P. Automated system for the on-line monitoring of powder blending processes using near-infrared spectroscopy. Part II. Qualitative approaches to blend evaluation. J Pharm Biomed Anal. 1998;17(8):1285–309. https://doi.org/10.1016/S0731-7085(98)00025-9.
Besseling R, Damen M, Tran T, Nguyen T, Dries K, Oostra W, Gerich A. An efficient, maintenance free and approved method for spectroscopic control and monitoring of blend uniformity: the moving F-test. J Pharm Biomed Anal. 2015;114:471–81. https://doi.org/10.1016/j.jpba.2015.06.019.
Wargo D, Drennen J. Near-infrared spectroscopic characterization of pharmaceutical powder blends. J Pharm Biomed Anal. 1996;14(11):1415–23. https://doi.org/10.1016/0731-7085(96)01739-6.
Blanco M, Bañó RG, Bertran E. Monitoring powder blending in pharmaceutical processes by use of near infrared spectroscopy. Talanta. 2002;56(1):203–12. https://doi.org/10.1016/S0039-9140(01)00559-8.
Sánchez FC, Toft J, Bogaert B, Massart D, Dive S, Hailey P. Monitoring powder blending by NIR spectroscopy. Fresenius J Anal Chem. 1995;352:771–8. https://doi.org/10.1007/BF00323062.
Puchert T, Holzhauer C, Menezes J, Lochmann D, Reich G. A new PAT/QbD approach for the determination of blend homogeneity: combination of online NIRS analysis with PC scores distance analysis (PC-SDA). Eur J Pharm Biopharm. 2011;78(1):173–82. https://doi.org/10.1016/j.ejpb.2010.12.015.
Plugge W, Vlies C. Near-infrared spectroscopy as an alternative to assess compliance of ampicillin trihydrate with compendial specifications. J Pharm Biomed Anal. 1993;11:435–42. https://doi.org/10.1016/0731-7085(93)80154-S.
Storme-Paris I, Clarot I, Esposito S, Chaumeil J, Nicolas A, Brion F, et al. Near infrared spectroscopy homogeneity evaluation of complex powder blends in a small-scale pharmaceutical preformulation process, a real-life application. Eur J Pharm Biopharm. 2009;72:189–98. https://doi.org/10.1016/j.ejpb.2008.11.002.
Beer T, Bodson C, Dejaegher B, Walczak B, Vercruysse P, Burggraeve A, et al. Raman spectroscopy as a process analytical technology (PAT) tool for the in-line monitoring and understanding of a powder blending process. J Pharm Biomed Anal. 2008;48:772–9. https://doi.org/10.1016/j.jpba.2008.07.023.
El-Hagrasy AS, Delgado-Lopez M, Drennen JK. A process analytical technology approach to near-infrared process control of pharmaceutical powder blending: Part II: qualitative near-infrared models for prediction of blend homogeneity. J Pharm Sci. 2006;95:407–21. https://doi.org/10.1002/jps.20466.
Gerich A, Verhoog J, Damen M, Verhoeven W, Chamarthy S, Besseling R. Detection of lumps in powder blends by inline NIR, Pharmaceutical Technology, Pharmaceutical Technology. 2017; 41(9): 36–44. https://www.pharmtech.com/view/detection-lumps-powder-blends-inline-nir
Wu H, Tawakkul M, White M, Khan M. Quality-by-design (QbD): an integrated multivariate approach for the component quantification in powder blends. Int J Pharm. 2009;372:39–48. https://doi.org/10.1016/j.ijpharm.2009.01.002.
Chavez PZ, Sacré PY, De Bleye C, Netchacovitch L, Mantanus J, Motte H, Schubert M, Hubert P, Ziemons E. Active content determination of pharmaceutical tablets using near infrared spectroscopy as process analytical technology tool. Talanta. 2015;144:1352–9. https://doi.org/10.1016/j.talanta.2015.08.018.
Nakagawa H, Kano M, Hasebe S, Miyano T, Watanabe T, Wakiyama N. Verification of model development technique for NIR-based real-time monitoring of ingredient concentration during blending. Int J Pharm. 2014;471:264–75. https://doi.org/10.1016/j.ijpharm.2014.05.013.
Jaumot J, Igne B, Anderson C, Drennen J, de Juan A. Blending process modeling and control by multivariate curve resolution. Talanta. 2013;117:492–504. https://doi.org/10.1016/j.talanta.2013.09.037.
Martínez L, Peinadob A, Liesum L. In-line quantification of two active ingredients in a batch blending process by near-infrared spectroscopy: influence of physical presentation of the sample. Int J Pharm. 2013;451:67–75. https://doi.org/10.1016/j.ijpharm.2013.04.078.
Alam MA, Liu Y. An agile and robust in-line NIR potency deviation detection method for monitoring and control of a continuous direct compression process. Int J Pharm. 2021;601:120521. https://doi.org/10.1016/j.ijpharm.2021.12052.
Mohan S, Momose W, Katz J, Hossain MN, Velez N, Drennen J, Anderson C. A robust quantitative near infrared modeling approach for blend monitoring. J Pharm Biomed Anal. 2018;148:51–7. https://doi.org/10.1016/j.jpba.2017.09.011.
Rinnan A, van den Berg F, Engelsen S. Review of the most common pre-processing techniques for near-infrared spectra. Trends Anal Chem. 2009;28(10):1201–22. https://doi.org/10.1016/j.trac.2009.07.007.
Barnes R, Dhanoa M, Lister S. Standard normal variate transformation and de-trending of near–infrared diffuse reflectance spectra. Appl Spectrosc. 1989;43(5):772–7. https://doi.org/10.1366/0003702894202201.
Mark H, Workman J. Derivatives in spectroscopy. Spectrosc. 2003;18(4):37.
Cowe I, McNicol J. The use of principal components in the analysis of near-infrared spectra. Appl. Spectrosc. 1985; 39: 257–266. https://opg.optica.org/as/abstract.cfm?URI=as-39-2-257
Wold S, Esbensen K, Geladi P. Principal component analysis. Chemom Intell Lab Syst. 1987;2:37–52. https://doi.org/10.1016/0169-7439(87)80084-9.
Gerlach R, Kowalski B, Wold H. Partial least-squares path modelling with latent variables. Anal Chim Acta. 1979;112(4):417–21. https://doi.org/10.1016/S0003-2670(01)85039-X.
Wold S, Sjostrom M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemom Intell Lab Syst. 2001;58:109–30. https://doi.org/10.1016/S0169-7439(01)00155-1.
De Bleye C, Chavez P, Mantanus J, Marini R, Hubert P, Rozet E, Ziemons E. Critical review of near-infrared spectroscopic methods validations in pharmaceutical applications. J Pharm Biomed Anal. 2012;69:125–32. https://doi.org/10.1016/j.jpba.2012.02.003.
US Food and Drug Association Draft guideline-powder blends and finished dosage units — stratified in-process dosage unit sampling and assessment 2003. https://pqri.org/wp-content/uploads/2015/12/FDADraftGuide.pdf
Brone D, Muzzio F. Enhanced mixing in double-cone blenders. Powder Technol. 2000;110:179–89. https://doi.org/10.1016/S0032-5910(99)00204-1.
Barling D, Morton D, Hapgood K. Pharmaceutical dry powder blending and scale-up: maintaining equivalent mixing conditions using a coloured tracer powder. Powder Technol. 2015;270:461–9. https://doi.org/10.1016/j.powtec.2014.04.069.
Acknowledgements
The authors are thankful to the manufacturing team for helping in performing the trials.
Author information
Authors and Affiliations
Contributions
Aruna Khanolkar designed the experiments, interpreted the analysis, supervised the project, and modified the manuscript. Bhaskar Patil performed the experiments, analyzed the spectra data, and wrote the original manuscript. Viraj Thorat performed the experiments and analyzed the experimental spectral data. Gautam Samanta was responsible for supervision, review, and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khanolkar, A., Patil, B., Thorat, V. et al. Development of Inline Near-Infrared Spectroscopy Method for Real-Time Monitoring of Blend Uniformity of Direct Compression and Granulation-Based Products at Commercial Scales. AAPS PharmSciTech 23, 235 (2022). https://doi.org/10.1208/s12249-022-02392-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02392-9