Abstract
The present study aims to develop carboplatin injectable microspheres using spray-drying technology. The optimized powdered microspheres (MS-19-ST2) were morphologically spherical, with a 1.795 μm particle size and good micromeritic properties. Under normal temperature conditions, the MS-19-ST2 formulation exhibited a sustained release behaviour following first-order drug release kinetics with no compatibility issues with aluminium syringes. Furthermore, MS-19-ST2 formulation outperformed its commercial counterpart in terms of in vivo pharmaco-kinetics and -dynamics (MRT-13.9 ± 0.9 h, T1/2–8.2 ± 0.3 h, tumour inhibition-74.5%). Additionally, the MS-19-ST2 formulation was much safer to use than its commercial counterpart, as observed from the results of ex vivo (haemolytic, MTT, and cell apoptosis assays) and in vivo (14-day acute and 28-day sub-acute) toxicity studies. The above results confirm the MS-19-ST2 formulation as a good candidate to commercialize carboplatin in a powdered microsphere form (stable for 24 h after reconstitution) with improved pharmacokinetics, therapeutic, and toxicity profile.
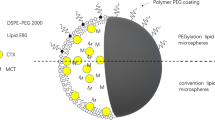
Graphical Abstract










Similar content being viewed by others
References
Newton HB. Neurological complications of chemotherapy to the central nervous system. Handb Clin Neurol Elsevier; 2012, 903–16.
Ali AA, McCrudden CM, McCarthy HO. Evaluation of the impact of nitric oxide on resistance to platinum-based chemotherapeutics. 1st ed. Nitric oxide as a Chemosensitizing agent. Elsevier Inc.; 2017.
Hang Z, Cooper MA, Ziora ZM. Platinum-based anticancer drugs encapsulated liposome and polymeric micelle formulation in clinical trials. Biochem Compd. Herbert Publications PVT LTD. 2016;4:1.
Carboplatin - an overview | ScienceDirect Topics [Internet]. [cited 2021 Jul 29]. Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/carboplatin
Khan MA, Zafaryab M, Mehdi SH, Quadri J, Rizvi MMA. Characterization and carboplatin loaded chitosan nanoparticles for the chemotherapy against breast cancer in vitro studies. Int J Biol Macromol Elsevier. 2017;97:115–22.
Poy D, Shahemabadi HE, Akbarzadeh A, Moradi-Sardareh H, Ebrahimifar M. Carboplatin liposomal nanoparticles: preparation, characterization, and cytotoxicity effects on lung cancer in vitro environment. https://doi.org/10.1080/0091403720171332624. Taylor & Francis 2017;67:367–70.
Harsha S, Al-Khars M, Al-Hassan M, Kumar NP, Nair AB, Attimarad M, et al. Pharmacokinetics and tissue distribution of spray-dried carboplatin microspheres: lung targeting via intravenous route. Arch Pharmacal Res, Springer. 2013;37:352–60.
Hamelers IHL, de Kroon, AIPM. Nanocapsules of platinum-based anticancer drugs. Platin Other Heavy Met Compd Cancer Chemother. Humana Press, Totowa, NJ; 2009;27–32.
Biswas S, Kumari P, Lakhani PM, Ghosh B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci Elsevier; 2016, 184–202.
Thakur S, Singh H, Singh A, Kaur S, Sharma A, Singh SK, et al. Thermosensitive injectable hydrogel containing carboplatin loaded nanoparticles: a dual approach for sustained and localized delivery with improved safety and therapeutic efficacy. J Drug Deliv Sci Technol Elsevier. 2020;58:101817.
Gao P, Xu H, Ding P, Gao Q, Sun J, Chen D. Controlled release of huperzine a from biodegradable microspheres: in vitro and in vivo studies. Int J Pharm. 2007;330:1–5.
Rajput MS, Agrawal P. Microspheres in cancer therapy. Indian J Cancer Medknow Publications and Media Pvt Ltd; 2010, 458–68.
Aurea Biolabs Zeal technology™. 2018.
Manunta ML, Gavini E, Chessa G, Passino ES, Careddu GM, Giua S, et al. Carboplatin sustained delivery system using injectable microspheres. J Vet Med Ser A Physiol Pathol Clin Med. 2005;52:416–22.
Lu B, Zhang JQ, Yang H. Lung-targeting microspheres of carboplatin. Int J Pharm Elsevier. 2003;265:1–11.
Gavini E, Manunta L, Giua S, Achenza G, Giunchedi P. Spray-dried poly(D,L-lactide) microspheres containing carboplatin for veterinary use: In vitro and in vivo studies. AAPS PharmSciTech Springer. 2005;6:E108–14.
Biomaterials - Physics and Chemistry - New Edition. Biomater. - Phys. Chem. - New Ed. 2018.
Al-Khattawi A, Bayly A, Phillips A, Wilson D. The design and scale-up of spray dried particle delivery systems. Expert Opin Drug Deliv Taylor & Francis; 2018, 47–63.
Gaspar F. Spray drying in the pharmaceutical industry [internet]. Eur Pharm Rev 2014 [cited 2021 Oct 21]. Available from: https://www.europeanpharmaceuticalreview.com/article/27768/spray-drying-pharmaceutical-industry/
Mo J, Eggers PK, Chen X, Ahamed MRH, Becker T, Lim LY, et al. Shear induced carboplatin binding within the cavity of a phospholipid mimic for increased anticancer efficacy. Sci Rep Nature Publishing Group. 2015;5:1–9.
Hines DJ, Kaplan DL. Poly(lactic-co-glycolic) Acid-controlled-release systems: experimental and modeling insights. Crit Rev Ther Drug Carrier Syst. NIH public Access. 2013;30:257–76.
Yermak IM, Khotimchenko YS. Chemical properties, biological activities and applications of carrageenans from red algae. Recent Adv Mar Biotechnol. 2003;9:207–55.
Beg S, Rahman M, Panda SK, Alharbi KS, Alruwaili NK, Ameeduzzafar, et al. Nasal Mucoadhesive microspheres of Lercanidipine with improved systemic bioavailability and antihypertensive activity. J Pharm Innov. 2021;16:237–46.
He T, Wang W, Chen B, Wang J, Liang Q, Chen B. 5-fluorouracil monodispersed chitosan microspheres: microfluidic chip fabrication with crosslinking, characterization, drug release and anticancer activity. Carbohydr Polym Elsevier. 2020;236:116094.
Mukhi S, Bose A, Panda P, Rao MM. Pharmacognostic, physicochemical and chromatographic characterization of Samasharkara Churna. J Ayurveda Integr Med Elsevier BV. 2016;7:88–99.
Agrawal GR, Wakte P, Shelke S. Formulation, physicochemical characterization and in vitro evaluation of human insulin-loaded microspheres as potential oral carrier. Prog Biomater Springer. 2017;6:125–36.
Singh H, Kumar C, Singh N, Paul S, Jain SK. Nanoencapsulation of docosahexanoic acid (DHA) using combination of food grade polymeric wall materials and its application for improvement in bioavailability and oxidative stability. Food Funct Food Funct. 2018;9:2213–27.
Singh H, Nathani S, Singh N, Roy P, Paul S, Sohal HS, et al. Development and characterization of solid-SNEDDS formulation of DHA using hydrophilic carrier with improved shelf life, oxidative stability and therapeutic activity. J Drug Deliv Sci Technol 2019;54.
Carboplatin Leaflet [Internet]. [cited 2021 Oct 21]. Available from: https://www.bing.com/search?q=Aluminium+%2B+Carboplatin+%2B+incompatibility&qs=n&form=QBRE&sp=-1&pq=aluminium+%2B+carboplatin+%2B+incompati&sc=0-35&sk=&cvid=CCF31F2A1EBA4B8F80A5A85EBD63BC2A
Although USFDA, Fda TUS. In Vitro Hemolysis Assay. :1–5.
Thakur S, Singh H, Singh A, Kaur S, Sharma A, Singh SK, et al. Thermosensitive injectable hydrogel containing carboplatin loaded nanoparticles: a dual approach for sustained and localized delivery with improved safety and therapeutic efficacy. J Drug Deliv Sci Technol Editions de Sante 2020;58.
Kumar A, Kaur V, Pandit K, Tuli HS, Sak K, Jain SK, et al. Antioxidant Phytoconstituents from Onosma bracteata wall. (Boraginaceae) ameliorate the CCl4 induced hepatic damage: In Vivo study in male Wistar rats. Front Pharmacol. Frontiers Media S.A.; 2020, 11.
Singh A, Thakur S, Singh H, Singh H, Kaur S, Kaur S, et al. Novel Vitamin E TPGS based docetaxel nanovesicle formulation for its safe and effective parenteral delivery: Toxicological, pharmacokinetic and pharmacodynamic evaluation. J Liposome Res. Taylor and Francis Ltd. 2021;31:365–80.
Singh A, Thakur S, Singh N, Kaur S, Jain SK. Novel Gellan Gum-Based In Situ Nanovesicle Formulation of Docetaxel for Its Localized Delivery Using Depot Formation. AAPS PharmSciTech. Springer Science and Business Media Deutschland GmbH; 2021;22.
Guidelines O. OECD/OCDE 423 OECD GUIDELINE FOR TESTING OF CHEMICALS acute Oral toxicity-acute toxic class method INTRODUCTION. 2001.
OECD Guideline For The Testing of Chemicals Adopted by the Council on 27 th July 1995 Repeated Dose 28-day Oral Toxicity Study in Rodents.
Drugs.com. Carboplatin Dosage Guide + Max Dose, Adjustments - Drugs.com [Internet]. 2021 [cited 2021 Oct 28]. Available from: https://www.drugs.com/dosage/carboplatin.html
Singh H, Sahajpal NS, Singh H, Vanita V, Roy P, Paul S, et al. Pre-clinical and cellular toxicity evaluation of 7-methylxanthine: an investigational drug for the treatment of myopia. Drug Chem Toxicol 2019
Harsha S, Al-Khars M, Al-Hassan M, Kumar NP, Nair AB, Attimarad M, et al. Pharmacokinetics and tissue distribution of spray-dried carboplatin microspheres: lung targeting via intravenous route. Arch Pharm Res Arch Pharm Res. 2014;37:352–60.
Gavini E, Manunta L, Giua S, Achenza G, Giunchedi P. Spray-dried poly(D,L-lactide) microspheres containing carboplatin for veterinary use: In vitro and in vivo studies. AAPS PharmSciTech. AAPS PharmSciTech; 2005;6.
de Barros C, Aranha N, Severino P, Souto EB, Zielińska A, Lopes A, et al. Quality by design approach for the development of liposome carrying ghrelin for intranasal administration. Pharmaceutics. Multidisciplinary Digital Publishing Institute (MDPI); 2021;13.
Habtegebriel H, Wawire M, Sila D. The effect of pretreatment (spray drying) on the yield and selected nutritional components of whole camel Milk powder. J Food Sci J Food Sci. 2018;83:2983–91.
Maheshwari R, Todke P, Kuche K, Raval N, Tekade RK. Micromeritics in pharmaceutical product development. Dos Form Des Considerations. 2018;I:599–635.
Lucideon. BET Surface Area Analysis & BJH Pore Size & Volume Analysis Technique [Internet]. [cited 2021 Oct 28]. Available from: https://www.lucideon.com/testing-characterization/techniques/bet-surface-area-analysis-bjh-pore-size-volume-analysis
Jaworska A, Wojcik T, Malek K, Kwolek U, Kepczynski M, Ansary AA, et al. Rhodamine 6G conjugated to gold nanoparticles as labels for both SERS and fluorescence studies on live endothelial cells. Microchim Acta. 2015;182:119–27.
Acknowledgments
This project was supported by the University Grants Commission (UGC) under RUSA 2.0 Component 4 Scheme provided to Guru Nanak Dev University, Amritsar (Prof. (Dr.) Subheet Kumar Jain, Principal-Investigator). We are also thankful for providing a grant under the DST-FIST scheme to the Department of Pharmaceutical Sciences, Guru Nanak Dev University, Amritsar.
Author information
Authors and Affiliations
Contributions
Shubham Thakur contributed to conceptualization, methodology, investigation, validation, formal analysis, writing—original draft, visualization; Rasdeep Kaur contributed to methodology, investigation, validation, formal analysis; Satwinderjeet Kaur contributed to conceptualization, supervision, writing—review and editing; Subheet Kumar Jain contributed to conceptualization, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thakur, S., Kour, R., Kaur, S. et al. Spray-Dried Microspheres of Carboplatin: Technology to Develop Longer-Acting Injectable with Improved Physio-Chemical Stability, Toxicity, and Therapeutics. AAPS PharmSciTech 23, 128 (2022). https://doi.org/10.1208/s12249-022-02281-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02281-1