Abstract
Piperine (PIP) was evaluated as a natural coformer in the preparation of multicomponent organic materials for enhancing solubility and dissolution rate of the poorly water-soluble drugs: curcumin (CUR), lovastatin (LOV), and irbesartan (IBS). A screening based on liquid assisted grinding technique was performed using 1:1 drug-PIP molar ratio mixtures, followed by differential scanning calorimetry (DSC) and powder X-ray diffraction (PXRD) analyses. Three eutectic mixtures (EMs) composed of CUR-PIP, LOV-PIP, and IBS-PIP were obtained. Therefore, binary phase and Tamman’s diagrams were constructed for each system to obtain the exact eutectic composition, which was 0.41:0.59, 0.29:0.71, and 0.31:0.69 for CUR-PIP, LOV-PIP, and IBS-PIP, respectively. Further, bulk materials of each system were prepared to characterize them through DSC, PXRD fully, Fourier transform infrared spectroscopy (FT-IR), and solution-state nuclear magnetic resonance (NMR) spectroscopy. In addition, the contact angle, solubility, and dissolution rate of each system were evaluated. The preserved characteristic in the PXRD patterns and FT-IR spectra of the bulk material of each system confirmed the formation of EM mixture without molecular interaction in solid-state. The formation of EM resulted in improved aqueous solubility and dissolution rate associated with the increased wettability observed by the decrease in contact angle. In addition, solution NMR analyses of CUR-PIP, LOV-PIP, and IBS-PIP suggested no significant intermolecular interactions in solution between the components of the EM. Hence, this study concludes that PIP could be an effective coformer to improve the solubility and dissolution rate of CUR, LOV, and IBS.
Graphical Abstract













Similar content being viewed by others
References
World-Health-Organization. Health statistics and information systems Disease burden and mortality estimates [Internet]. 2020 [cited 2021 Feb 1]. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates
Castillo-Rivas J. Atención de la enfermedades cardiovasculares en la Caja Costarricense de Seguro Social:: 1998 -2005. Scielo. 2006.
Luengo-Fernandez R. Cost of cardiovascular diseases in the United Kingdom. Heart. 2006;92:1384–9.
Pérez-Gonzales K. CCSS invierte ¢245.350 millones en atención de pacientes con enfermedades cardiovasculares. 2017. San José, Costa Rica; 2017;
Cooper R, Cutler J, Desvigne-Nickens P, Fortmann SP, Friedman L, Havlik R, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States. Circulation. 2000;102:3137–47.
Andreassen M, Raymond I, Kistorp C, Hildebrandt P, Faber J, Kristensen LØ. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur J Endocrinol. 2009;160:25–31.
Li H, Sureda A, Devkota HP, Pittalà V, Barreca D, Silva AS, et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv. 2020;38:107343.
Zolkiflee NF, Affandi MMRMM, Majeed ABA. Thermodynamics and solute-solvent interactions of lovastatin in an aqueous arginine solution. Eur J Pharm Sci. 2020;141:105111.
Leve S, Banga H, Shankar P, Dixit R. An experimental study of a novel combination of a herbal drug with an allopathic drug to evaluate the antihyperglycemic effect of irbesartan plus curcumin and comparison with glibenclamide. Int J Basic Clin Pharmacol. 2013;2:182.
Campbell MS, Fleenor BS. The emerging role of curcumin for improving vascular dysfunction: a review. Crit Rev Food Sci Nutr. 2018;58:2790–9.
Salehi B, Del Prado-Audelo ML, Cortés H, Leyva-Gómez G, Stojanović-Radić Z, Singh YD, et al. Therapeutic applications of curcumin nanomedicine formulations in cardiovascular diseases. J Clin Med. 2020;9:746.
Song Y, Chen J, Sun M, Gong C, Shen Y, Song Y, et al. A simple electrochemical biosensor based on AuNPs/MPS/Au electrode sensing layer for monitoring carbamate pesticides in real samples. J Hazard Mater. 2016;
Bi X, Yuan Z, Qu B, Zhou H, Liu Z, Xie Y. Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2. Phytomedicine. 2019;54:98–108.
Zhang B, Yang J, Qin Z, Li S, Xu J, Yao Z, et al. Mechanism of the efflux transport of demethoxycurcumin-O-glucuronides in HeLa cells stably transfected with UDP-glucuronosyltransferase 1A1. Hsieh Y-H, editor. PLoS One. 2019;14:e0217695.
Phansalkar PS, Zhang Z, Verenich S, Gerk PM. Pharmacokinetics and bioavailability enhancement of natural products. Nat Prod Cancer Chemoprevention. Cham: Springer International Publishing; 2020. p. 109–41.
Wang L, Sun R, Zhang Q, Luo Q, Zeng S, Li X, et al. An update on polyphenol disposition via coupled metabolic pathways. Expert Opin Drug Metab Toxicol. 2019;15:151–65.
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–49.
Araya-Sibaja AM, Fandaruff C, Wilhelm K, Vega-Baudrit JR, Guillén-Girón T, Navarro-Hoyos M. Crystal engineering to design of solids: from single to multicomponent organic materials. Mini Rev Org Chem [Internet]. 2020;17:518–38. Available from: http://www.eurekaselect.com/171925/article
Vippagunta SR, Wang Z, Hornung S, Krill SL. Factors affecting the formation of eutectic solid dispersions and their dissolution behavior. J Pharm Sci [Internet]. 2007;96:294–304. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022354916321943
Kumar S, Nanda A. Approaches to design of pharmaceutical cocrystals: a review. Mol Cryst Liq Cryst. 2018;667:54–77.
Rathi N, Paradkar A, Gaikar VG. Polymorphs of curcumin and its cocrystals with cinnamic acid. J Pharm Sci. 2019;108:2505–16.
Sathisaran I, Dalvi SV. Crystal engineering of curcumin with salicylic acid and hydroxyquinol as coformers. Cryst Growth Des [Internet]. 2017;17:3974–88. Available from: http://pubs.acs.org/doi/https://doi.org/10.1021/acs.cgd.7b00599
Suresh K, Nangia A. Curcumin: pharmaceutical solids as a platform to improve solubility and bioavailability. CrystEngComm [Internet]. 2018; Available from: http://xlink.rsc.org/?DOI=C8CE00469B
Wang R, Han J, Jiang A, Huang R, Fu T, Wang L, et al. Involvement of metabolism-permeability in enhancing the oral bioavailability of curcumin in excipient-free solid dispersions co-formed with piperine. Int J Pharm [Internet]. 2019;561:9–18. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378517319301504
Singh J, Dubey RK, Atal CK. Piperine-mediated inhibition of glucuronidation activity in isolated epithelial cells of the guinea-pig small intestine: evidence that piperine lowers the endogeneous UDP-glucuronic acid content. J Pharmacol Exp Ther. 1986;236.
FDA. CFR - Code of Federal Regulations Title 21-Food aditives permitted for direct addition to food for human consumptions.
Chadha K, Karan M, Chadha R, Bhalla Y, Vasisht K. Is failure of cocrystallization actually a failure? Eutectic formation in cocrystal screening of hesperetin. J Pharm Sci [Internet]. 2017;106:2026–36. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022354917302745
Araya-Sibaja A, Vega-Baudrit J, Guillén-Girón T, Navarro-Hoyos M, Cuffini S. Drug solubility enhancement through the preparation of multicomponent organic materials: eutectics of lovastatin with carboxylic acids. Pharmaceutics [Internet]. 2019;11:112. Available from: https://www.mdpi.com/1999-4923/11/3/112
Rycerz L. Practical remarks concerning phase diagrams determination on the basis of differential scanning calorimetry measurements. J Therm Anal Calorim [Internet]. 2013;113:231–8. Available from: http://link.springer.com/https://doi.org/10.1007/s10973-013-3097-0
Höhne GWH, Hemminger WF, Flammersheim H-J. Differential scanning calorimetry [Internet]. Berlin, Heidelberg: Springer Berlin Heidelberg; 2003. Available from: http://link.springer.com/https://doi.org/10.1007/978-3-662-06710-9
Gaisford S, Kett V, Haines P, editors. Principles of thermal analysis and calorimetry. The Royal Society of Chemistry; 2016.
Boettinger WJ, Kattner UR, Moon K-W, Perepezko JH. Methods for phase diagram determination [Internet]. Zhao J-C, editor. Elsevier; 2007. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780080446295X50009
FDA. Guidance for industry dissolution testing of immediate solid oral dosage form [Internet]. 1997. Available from: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070246.pdf
Swanepoel E, Liebenberg W, de Villiers MM. Quality evaluation of generic drugs by dissolution test: changing the USP dissolution medium to distinguish between active and non-active mebendazole polymorphs. Eur J Pharm Biopharm [Internet]. 2003;55:345–9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0939641103000043
Honorato SB, Farfan S, Viana A, Filho JM, Camar??o GC, Fechine F V., et al. Polymorphism evaluation in generic tablets containing mebendazole by dissolution tests. J Braz Chem Soc. 2012;23:220–7.
Moorthi C, Senthil Kumar C, Mohan S, Krishnan K, Kathiresan K. Application of validated RP–HPLC–PDA method for the simultaneous estimation of curcumin and piperine in Eudragit E 100 nanoparticles. J Pharm Res [Internet]. 2013;7:224–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S097469431300114X
PubChem. Lovastatin Water Solubility [Internet]. [cited 2021 Feb 1]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Lovastatin#section=Solubility&fullscreen=true
PubChem. Irbesartan Water Solubility [Internet]. [cited 2021 Aug 21]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Irbesartan#section=Solubility&fullscreen=true
PubChem. Curcumin Water Solubility [Internet]. [cited 2021 Jan 10]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Curcumin#section=Solubility&fullscreen=true
Martins GS. Differential Scanning Thermal Analysis of Shape-Memory Polymers, Polymer Blends and Composites. 2020. p. 153–66.
Chaturvedi K, Shah HS, Nahar K, Dave R, Morris KR. Contribution of crystal lattice energy on the dissolution behavior of eutectic solid dispersions. ACS Omega. 2020;5:9690–701.
Lin B, Liu Y, Wang M, Wang Y, Du S, Gong J, et al. Intermolecular interactions and solubility behavior of multicomponent crystal forms of orotic acid: prediction and experiments. Cryst Growth Des. 2021;21:1473–81.
Moore MD, Wildfong PLD. Aqueous solubility enhancement through engineering of binary solid composites: pharmaceutical applications. J Pharm Innov. 2009;4:36–49.
Jackson KA, Hunt JD. Lamellar and rod eutectic growth. Dyn Curved Front. Elsevier; 1988. p. 363–76.
Jain H, Khomane KS, Bansal AK. Implication of microstructure on the mechanical behaviour of an aspirin–paracetamol eutectic mixture. CrystEngComm [Internet]. 2014;16:8471–8. Available from: http://xlink.rsc.org/?DOI=C4CE00878B
Fiandaca M, Dalwadi G, Wigent R, Gupta P. Ionic liquid formation with deep eutectic forces at an atypical ratio (2:1) of naproxen to lidocaine in the solid-state, thermal characterization and FTIR investigation. Int J Pharm. 2020;575:118946.
Meira RZC, Biscaia IFB, Nogueira C, Murakami FS, Bernardi LS, Oliveira PR. Solid-state characterization and compatibility studies of penciclovir, lysine hydrochloride, and pharmaceutical excipients. Materials (Basel). 2019;12:3154.
Gandolfo FG, Bot A, Flöter E. Phase diagram of mixtures of stearic acid and stearyl alcohol. Thermochim Acta. 2003;404:9–17.
Patel RD, Raval MK, Sheth NR. Formation of diacerein − fumaric acid eutectic as a multi-component system for the functionality enhancement. J Drug Deliv Sci Technol. 2020;58:101562.
Hyun S-M, Lee BJ, Abuzar SM, Lee S, Joo Y, Hong S-H, et al. Preparation, characterization, and evaluation of celecoxib eutectic mixtures with adipic acid/saccharin for improvement of wettability and dissolution rate. Int J Pharm [Internet]. 2019;554:61–71. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378517318307841
Leitner J, Jurik S. DSC study and thermodynamic modelling of the system paracetamol–o-acetylsalicylic acid. J Therm Anal Calorim [Internet]. 2017;130:1735–40. Available from: http://link.springer.com/https://doi.org/10.1007/s10973-017-6404-3
Dichi E, Sghaier M, Guiblin N. Reinvestigation of the paracetamol–caffeine, aspirin–caffeine, and paracetamol–aspirin phase equilibria diagrams. J Therm Anal Calorim [Internet]. 2018;131:2141–55. Available from: http://link.springer.com/https://doi.org/10.1007/s10973-017-6855-6
Rathod MK, Banerjee J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew Sustain Energy Rev. 2013;18:246–58.
Das SS, Singh NP, Agrawal T, Gupta P, Tiwari SN, Singh NB. Studies of solidification behavior and molecular interaction in benzoic acid–o-chloro benzoic acid eutectic system. Mol Cryst Liq Cryst. 2009;501:107–24.
Li W, Shi P, Jia L, Zhao Y, Sun B, Zhang M, et al. Eutectics and salt of dapsone with hydroxybenzoic acids: binary phase diagrams, characterization and evaluation. J Pharm Sci. 2020;
Larkin P. Environmental dependence of vibrational spectra. Infrared Raman Spectrosc [Internet]. Elsevier; 2011. p. 55–62. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780123869845100047
Taylor R, Wood PA. A million crystal structures: the whole is greater than the sum of its parts. Chem Rev [Internet]. 2019;119:9427–77. Available from: https://pubs.acs.org/doi/https://doi.org/10.1021/acs.chemrev.9b00155
Desiraju GR. Crystal engineering: from molecule to crystal. J Am Chem Soc [Internet]. 2013 [cited 2014 May 28];135:9952–67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23750552
Desiraju GR. Crystal engineering: a holistic view. Angew Chem Int Ed Engl [Internet]. 2007 [cited 2014 May 19];46:8342–56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17902079
Dahiya S, Rani R, Dhingra D, Kumar S, Dilbaghi N. Conjugation of epigallocatechin gallate and piperine into a zein nanocarrier: implication on antioxidant and anticancer potential. Adv Nat Sci Nanosci Nanotechnol. 2018;9:035011.
Zaini E, Afriyani A, Fitriani L, Ismed F, Horikawa A, Uekusa H. Improved solubility and dissolution rates in novel multicomponent crystals of piperine with succinic acid. Sci Pharm. 2020;88:21.
Górniak A, Gajda M, Pluta J, Czapor-Irzabek H, Karolewicz B. Thermal, spectroscopic and dissolution studies of lovastatin solid dispersions with acetylsalicylic acid. J Therm Anal Calorim. 2016;
Cherukuvada S, Nangia A. Eutectics as improved pharmaceutical materials: design, properties and characterization. Chem Commun [Internet]. 2014;50:906–23. Available from: http://xlink.rsc.org/?DOI=C3CC47521B
Park H, Seo HJ, Ha E-S, Hong S, Kim J-S, Kim M-S, et al. Preparation and characterization of glimepiride eutectic mixture with l-arginine for improvement of dissolution rate. Int J Pharm [Internet]. 2020;581:119288. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378517320302726
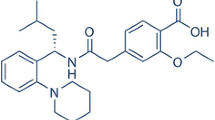
Araya-Sibaja AM, Maduro Campos CE, Fandaruff C, Vega-Baudrit JR, Guillén-Girón T, Hoyos MN, et al. Irbesartan desmotropes: Solid-state characterization, thermodynamic study and dissolution properties. J Pharm Anal [Internet]. 2019; Available from: https://linkinghub.elsevier.com/retrieve/pii/S2095177919300814
Pandey KU, Dalvi SV. Understanding stability relationships among three curcumin polymorphs. Adv Powder Technol [Internet]. 2019;30:266–76. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0921883118308392
Patel M, Tekade A, Gattani S, Surana S. Solubility enhancement of lovastatin by modified locust bean gum using solid dispersion techniques. AAPS PharmSciTech [Internet]. 2008 [cited 2014 Jun 4];9:1262–9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2628263&tool=pmcentrez&rendertype=abstract
Yoshida MI, Oliveira MA, Gomes ECL, Mussel WN, Castro W V., Soares CD V. Thermal characterization of lovastatin in pharmaceutical formulations. J Therm Anal Calorim [Internet]. 2011;106:657–64. Available from: http://link.springer.com/https://doi.org/10.1007/s10973-011-1510-0
Nicolaides E, Galia E, Efthymiopoulos C, Dressman JB, Reppas C. Forecasting the in vivo performance of four low solubility drugs from their in vitro dissolution data. Pharm. Res. 1999. p. 1876–82.
Zaborenko N, Shi Z, Corredor CC, Smith-Goettler BM, Zhang L, Hermans A, et al. First-principles and empirical approaches to predicting in vitro dissolution for pharmaceutical formulation and process development and for product release testing. AAPS J [Internet]. 2019;21:32. Available from: http://link.springer.com/https://doi.org/10.1208/s12248-019-0297-y
Dressman JB, Reppas C. In vitro–in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci [Internet]. 2000;11:S73–80. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0928098700001810
Gómez SM, Martínez JA, Martínez F. Validación de un método analítico empleando cromatografía líquida de alta eficiencia para la determinación de ibuprofeno en medios biorrelevantes. Quim Nova. 2010;33:1794–9.
Bhattachar SN, Risley DS, Werawatganone P, Aburub A. Weak bases and formation of a less soluble lauryl sulfate salt/complex in sodium lauryl sulfate (SLS) containing media. Int J Pharm. 2011;412:95–8.
Huang Z, Parikh S, Fish WP. Interactions between a poorly soluble cationic drug and sodium dodecyl sulfate in dissolution medium and their impact on in vitro dissolution behavior. Int J Pharm. 2018;535:350–9.
Guo Y, Wang C, Dun J, Du L, Hawley M, Sun CC. Mechanism for the reduced dissolution of ritonavir tablets by sodium lauryl sulfate. J Pharm Sci. 2019;108:516–24.
Fandaruff C, Rauber GS, Araya-Sibaja AM, Pereira RN, de Campos CEM, Rocha HVA, et al. Polymorphism of anti-HIV drug efavirenz: investigations on thermodynamic and dissolution properties. Cryst Growth Des [Internet]. 2014 [cited 2014 Nov 7];14:4968–75. Available from: http://pubs.acs.org/doi/abs/https://doi.org/10.1021/cg500509c
Fandaruff C, Segatto Silva MA, Galindo Bedor DC, de Santana DP, Rocha HVA, Rebuffi L, et al. Correlation between microstructure and bioequivalence in anti-HIV drug efavirenz. Eur J Pharm Biopharm [Internet]. 2015;91:52–8. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0939641115000363
Madhuri G, Nagaraju R, Killari KN. Enhancement of the physicochemical properties of poorly soluble lovastatin by co-crystallization techniques - in vivo studies. Indian J Pharm Sci [Internet]. 2020;82. Available from: https://www.ijpsonline.com/articles/enhancement-of-the-physicochemical-properties-of-poorly-soluble-lovastatin-by-cocrystallization-techniques--in-vivo-studies-3865.html
Rachmawati H, Edityaningrum CA, Mauludin R. Molecular inclusion complex of curcumin–β-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS PharmSciTech [Internet]. 2013;14:1303–12. Available from: http://link.springer.com/https://doi.org/10.1208/s12249-013-0023-5
Zhang Z, Le Y, Wang J, Zhao H, Chen J. Irbesartan drug formulated as nanocomposite particles for the enhancement of the dissolution rate. Particuology [Internet]. 2012 [cited 2014 May 14];10:462–7. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1674200112000296
Suryadevara V, Lankapalli S, Sunkara S, Sakhamuri V, Danda H. Dissolution rate enhancement of irbesartan and development of fast-dissolving tablets. Egypt Pharm J [Internet]. 2016;15:150. Available from: http://www.epj.eg.net/text.asp?2016/15/3/150/197583
Traxler F, Schinnerl J, Brecker L. Spectroscopic studies on the molecular interactions of curcumin and piperine. Monatshefte für Chemie - Chem Mon [Internet]. 2020;151:325–30. Available from: http://link.springer.com/https://doi.org/10.1007/s00706-020-02563-z
Kumar V, Mintoo MJ, Mondhe DM, Bharate SB, Vishwakarma RA, Bharate SS. Binary and ternary solid dispersions of an anticancer preclinical lead, IIIM-290: In vitro and in vivo studies. Int J Pharm [Internet]. 2019;570:118683. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378517319307288
Acknowledgements
Authors thank to Dr Oscar Rojas Carrillo and M. Sc. Marianelly Esquivel Alfaro for the support given in the use of the dissolution test system.
Funding
This contribution was partially funded by a grant from FEES-CONARE (Ref 115B9670). The financial support from the University of Costa Rica (UCR, Ref 115B6163), the Costa Rica Institute of Technology (TEC), the National Laboratory of Nanotechnology (LANOTEC CENAT), and the Technical University of Costa Rica (UTN).
Author information
Authors and Affiliations
Contributions
Conceptualization A.M.A.-S.; methodology, K.W., A.M.A.-S., F.V., M.Q. and M.N.-H.; formal analysis, K.W., F.V., M.Q.; investigation, K.W., A.M.A.-S., F.V., M.N.-H.; resources, T.G-G., J.R.V.-B., M.N.-H., A.M.A.-S.; data curation, K.W., A.M.A.-S., M.Q., F.V., M.N.-H.; writing—original draft preparation, K.W., A.M.A.-S., M.Q., F.V.; writing—review and editing, T.G-G., J.R.V.-B., M.N.-H., A.M.A.-S.; supervision, T.G-G., A.M.A.-S., J.R.V.-B., M.N.-H.; project administration, T.G-G., A.M.A.-S., M.N.-H.; funding acquisition, T.G-G., J.R.V.-B., M.N.-H. and A.M.A.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wilhelm-Romero, K., Quirós-Fallas, M.I., Vega-Baudrit, J.R. et al. Evaluation of Piperine as Natural Coformer for Eutectics Preparation of Drugs Used in the Treatment of Cardiovascular Diseases. AAPS PharmSciTech 23, 127 (2022). https://doi.org/10.1208/s12249-022-02270-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02270-4