Abstract
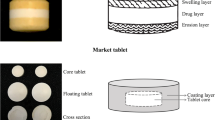
The objective of the present study was to develop microballoons aided gastro-retentive floating tablets of baclofen, a skeletal muscle relaxant with a low elimination half-life of ~ 3.5 h. Baclofen floating tablet was prepared to offer convenience by designing a tablet that would float in the stomach for a prolonged period and allow controlled drug release to enable once-a-day administration. Ethylcellulose microballoons (ECMBs) prepared by pseudo emulsion solvent diffusion method were employed as floating aid. The ECMBs were spherical with a size of 446.71 µm and a circularity index of 0.995. Buoyancy of 98.90 percent and good flowability reflected by an angle of repose of 23° suggested the feasibility of preparing floating tablets by direct compression. Directly compressed baclofen floating tablets comprised ECMBs, HPMC-K15M, and hydroxyl ethylcellulose as independent variables in the Box-Behnken design, however, performance characteristics of tablets such as in vitro drug release, floating lag time, and swelling index were selected as the dependent variables. Among the variables, ECMBs played a critical role in ensuring buoyancy. However, HPMC-K15M significantly influenced in vitro drug release. The optimized batch displayed Hickson-Crowell kinetics and exhibited a similar drug release profile as a marketed once-a-day formulation (f2, 91.03). Furthermore, optimized tablets showed a swelling index of > 300, floating lag time < 3 s, and total floating time > 24 h. Microballoons assisted floating tablets exhibited great promise for assured gastric retention of tablets.
GRAPHICAL ABSTRACT














Similar content being viewed by others
References
Sanchez-Ponce R, Wang LQ, Lu W, von Hehn J, Cherubini M, Rush R. Metabolic and pharmacokinetic differentiation of STX209 and racemic baclofen in humans. Metabolites. 2012;2(3):596–613. https://doi.org/10.3390/metabo2030596.
Sampat NG, Kulkarni RV, Sase N, Joshi NH, Vora PB, Bhattacharya AK, et al. Once daily baclofen sustained release or gastro-retentive system are acceptable alternatives to thrice daily baclofen immediate release at same daily dosage in patients. Neurol India. 2009;57(3):295–9. https://doi.org/10.4103/0028-3886.53284.
Whitehead L, Fell JT, Collett JH, Sharma HL, Smith A. Floating dosage forms: an in vivo study demonstrating prolonged gastric retention. J Control Release. 1998;55(1):3–12. https://doi.org/10.1016/s0168-3659(97)00266-6.
Klausner EA, Lavy E, Friedman M, Hoffman A. Expandable gastroretentive dosage forms. J Control Release. 2003;90(2):143–62. https://doi.org/10.1016/s0168-3659(03)00203-7.
Hoffman A, Stepensky D, Lavy E, Eyal S, Klausner E, Friedman M. Pharmacokinetic and pharmacodynamic aspects of gastroretentive dosage forms. Int J Pharm. 2004;277(1–2):141–53. https://doi.org/10.1016/j.ijpharm.2003.09.047.
Lopes CM, Bettencourt C, Rossi A, Buttini F, Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int J Pharm. 2016;510(1):144–58. https://doi.org/10.1016/j.ijpharm.2016.05.016.
Pawar VK, Kansal S, Asthana S, Chourasia MK. Industrial perspective of gastroretentive drug delivery systems: physicochemical, biopharmaceutical, technological and regulatory consideration. Expert Opin Drug Deliv. 2012;9(5):551–65. https://doi.org/10.1517/17425247.2012.677431.
Arora S, Ali J, Ahuja A, Khar RK, Baboota S. Floating drug delivery systems: a review. AAPS PharmSciTech. 2005;6(3):E372–90. https://doi.org/10.1208/pt060347.
Tadros MI. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: development, optimization and in vitro-in vivo evaluation in healthy human volunteers. Eur J Pharm Biopharm. 2010;74(2):332–9. https://doi.org/10.1016/j.ejpb.2009.11.010.
Qi X, Chen H, Rui Y, Yang F, Ma N, Wu Z. Floating tablets for controlled release of ofloxacin via compression coating of hydroxypropyl cellulose combined with effervescent agent. Int J Pharm. 2015;489(1–2):210–7. https://doi.org/10.1016/j.ijpharm.2015.05.007.
Yin L, Qin C, Chen K, Zhu C, Cao H, Zhou J, et al. Gastro-floating tablets of cephalexin: preparation and in vitro/in vivo evaluation. Int J Pharm. 2013;452(1–2):241–8. https://doi.org/10.1016/j.ijpharm.2013.05.011.
Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release. 2000;63(3):235–59. https://doi.org/10.1016/s0168-3659(99)00204-7.
Li S, Lin S, Daggy BP, Mirchandani HL, Chien YW. Effect of HPMC and carbopol on the release and floating properties of gastric floating drug delivery system using factorial design. Int J Pharm. 2003;253(1–2):13–22. https://doi.org/10.1016/s0378-5173(02)00642-7.
El-Zahaby SA, Kassem AA, El-Kamel AH. Design and evaluation of gastroretentive levofloxacin floating mini-tablets-in-capsule system for eradication of Helicobacter pylori. Saudi Pharm J. 2014;22(6):570–9. https://doi.org/10.1016/j.jsps.2014.02.009.
Gande S, Rao Y. Sustained-release effervescent floating matrix tablets of baclofen: development, optimization and in vitro-in vivo evaluation in healthy human volunteers. Daru. 2011;19(3):202–9.
Ranpise NS, Kolhe SR, Ranade AN. Development and evaluation of bilayer gastroretentive floating drug delivery system for baclofen. Indian J Pharm Educ Res. 2013;47(1):41–6.
Thakar K, Joshi G, Sawant KK. Bioavailability enhancement of baclofen by gastroretentive floating formulation: statistical optimization, in vitro and in vivo pharmacokinetic studies. Drug Dev Ind Pharm. 2013;39(6):880–8. https://doi.org/10.3109/03639045.2012.709249.
Gilbert DL Jr, Trissel LA, Martinez JF. Compatibility of ciprofloxacin lactate with sodium bicarbonate during simulated Y-site administration. Am J Health Syst Pharm . 1997;54(10):1193–5. https://doi.org/10.1093/ajhp/54.10.1193.
Trissel LA. Concentration-dependent precipitation of sodium bicarbonate with ciprofloxacin lactate. Am J Health Syst Pharm. 1996;53(1):84–5. https://doi.org/10.1093/ajhp/53.1.84.
Sinnott CJ, Garfield JM, Thalhammer JG, Strichartz GR. Addition of sodium bicarbonate to lidocaine decreases the duration of peripheral nerve block in the rat. Anesthesiology. 2000;93(4):1045–52. https://doi.org/10.1097/00000542-200010000-00028.
Korth-Bradley JM, Ludwig S. Incompatibility of amiodarone hydrochloride and sodium bicarbonate injections. Am J Health Syst Pharm. 1995;52(20):2340. https://doi.org/10.1093/ajhp/52.20.2340.
Acharya S, Patra S, Pani NR. Optimization of HPMC and carbopol concentrations in non-effervescent floating tablet through factorial design. Carbohydr Polym. 2014;102:360–8. https://doi.org/10.1016/j.carbpol.2013.11.060.
Kim S, Hwang KM, Park YS, Nguyen TT, Park ES. Preparation and evaluation of non-effervescent gastroretentive tablets containing pregabalin for once-daily administration and dose proportional pharmacokinetics. Int J Pharm. 2018;550(1–2):160–9. https://doi.org/10.1016/j.ijpharm.2018.08.038.
Kawashima Y, Niwa T, Handa T, Takeuchi H, Iwamoto T, Itoh K. Preparation of controlled-release microspheres of ibuprofen with acrylic polymers by a novel quasi-emulsion solvent diffusion method. J Pharm Sci. 1989;78(1):68–72. https://doi.org/10.1002/jps.2600780118.
Kawashima Y, Niwa T, Takeuchi H, Hino T, Ito Y. Preparation of multiple unit hollow microspheres (microballoons) with acrylic resin containing tranilast and their drug release characteristics (in vitro) and floating behavior (in vivo). J Control Release. 1991;16(3):279–89. https://doi.org/10.1016/0168-3659(91)90004-w.
Kawashima Y, Niwa T, Takeuchi H, Hino T, Itoh Y. Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J Pharm Sci. 1992;81(2):135–40. https://doi.org/10.1002/jps.2600810207.
Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. In vitro and in vivo evaluation of riboflavin-containing microballoons for a floating controlled drug delivery system in healthy humans. Int J Pharm. 2004;275(1–2):97–107. https://doi.org/10.1016/j.ijpharm.2004.01.036.
Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. In vivo evaluation of riboflavin-containing microballoons for floating controlled drug delivery system in healthy human volunteers. J Control Release. 2003;93(1):39–47. https://doi.org/10.1016/s0168-3659(03)00370-5.
Sato Y, Kawashima Y, Takeuchi H, Yamamoto H, Fujibayashi Y. Pharmacoscintigraphic evaluation of riboflavin-containing microballoons for a floating controlled drug delivery system in healthy humans. J Control Release. 2004;98(1):75–85. https://doi.org/10.1016/j.jconrel.2004.04.021.
Erni W, Held K. The hydrodynamically balanced system: a novel principle of controlled drug release. Eur Neurol. 1987;27(Suppl 1):21–7. https://doi.org/10.1159/000116171.
Bansal S, Beg S, Asthana A, Garg B, Asthana GS, Kapil R, et al. QbD-enabled systematic development of gastroretentive multiple-unit microballoons of itopride hydrochloride. Drug Deliv. 2016;23(2):437–51. https://doi.org/10.3109/10717544.2014.916771.
Malik P, Nagaich U, Malik RK, Gulati N. Pentoxifylline loaded floating microballoons: design, development and characterization. J Pharm (Cairo). 2013;2013: 107291. https://doi.org/10.1155/2013/107291.
Yadav A, Jain DK. Gastroretentive microballoons of metformin: formulation development and characterization. J Adv Pharm Technol Res. 2011;2(1):51–5. https://doi.org/10.4103/2231-4040.79806.
Kotagale NR, Parkhe AP, Jumde AB, Khandelwal HM, Umekar MJ. Ranitidine hydrochloride-loaded ethyl cellulose and Eudragit RS 100 buoyant microspheres: effect of pH modifiers. Indian J Pharm Sci. 2011;73(6):626–33. https://doi.org/10.4103/0250-474X.100236.
Yan E, Ding Y, Chen C, Li R, Hu Y, Jiang X. Polymer/silica hybrid hollow nanospheres with pH-sensitive drug release in physiological and intracellular environments. Chem Commun (Camb). 2009;19:2718–20. https://doi.org/10.1039/b900751b.
Cavalieri F, El Hamassi A, Chiessi E, Paradossi G. Stable polymeric microballoons as multifunctional device for biomedical uses: synthesis and characterization. Langmuir. 2005;21(19):8758–64. https://doi.org/10.1021/la050287j.
Manokruang K, Manias E. Hollow microspheres and aqueous phase behavior of pH-responsive poly(methyl methacrylate-co-methacrylic acid) copolymers with a blocky comonomer distribution. Mater Lett. 2009;63(13–14):1144–7. https://doi.org/10.1016/j.matlet.2009.01.046.
Jimenez-Martinez I, Quirino-Barreda T, Villafuerte-Robles L. Sustained delivery of captopril from floating matrix tablets. Int J Pharm. 2008;362(1–2):37–43. https://doi.org/10.1016/j.ijpharm.2008.05.040.
Dorozynski P, Jachowicz R, Kulinowski P, Kwiecinski S, Szybinski K, Skorka T, et al. The macromolecular polymers for the preparation of hydrodynamically balanced systems–methods of evaluation. Drug Dev Ind Pharm. 2004;30(9):947–57. https://doi.org/10.1081/ddc-200037179.
Jimenezcastellanos M, Zia H, Rhodes C. Design and testing in vitro of a bioadhesive and floating drug delivery system for oral application. Int J Pharm. 1994;105(1):65–70. https://doi.org/10.1016/0378-5173(94)90236-4.
Sinha Roy D, Rohera BD. Comparative evaluation of rate of hydration and matrix erosion of HEC and HPC and study of drug release from their matrices. Eur J Pharm Sci. 2002;16(3):193–9. https://doi.org/10.1016/s0928-0987(02)00103-3.
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, et al. Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta. 2007;597(2):179–86. https://doi.org/10.1016/j.aca.2007.07.011.
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76(5):965–77. https://doi.org/10.1016/j.talanta.2008.05.019.
Suryawanshi D, Wavhule P, Shinde U, Kamble M, Amin P. Development, optimization and in-vivo evaluation of cyanocobalamin loaded orodispersible films using hot-melt extrusion technology: a quality by design (QbD) approach. J Drug Deliv Sci Technol. 2021;63: 102559. https://doi.org/10.1016/j.jddst.2021.102559.
Escudero JJ, Ferrero C, Jimenez-Castellanos MR. Compaction properties, drug release kinetics and fronts movement studies from matrices combining mixtures of swellable and inert polymers: effect of HPMC of different viscosity grades. Int J Pharm. 2008;351(1–2):61–73. https://doi.org/10.1016/j.ijpharm.2007.09.031.
Lee PI, Peppas NA. Prediction of polymer dissolution in swellable controlled-release systems. J Control Release. 1987;6(1):207–15. https://doi.org/10.1016/0168-3659(87)90077-0.
Lee PI. Modeling of drug release from matrix systems involving moving boundaries: approximate analytical solutions. Int J Pharm. 2011;418(1):18–27. https://doi.org/10.1016/j.ijpharm.2011.01.019.
Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2001;48(2–3):139–57. https://doi.org/10.1016/s0169-409x(01)00112-0.
Acknowledgements
Authors are thankful to the University Grants Commission (UGC), Government of India, for providing fellowship to Pradipkumar Wavhule.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wavhule, P., Devarajan, P.V. Development and Optimization of Microballoons Assisted Floating Tablets of Baclofen. AAPS PharmSciTech 22, 272 (2021). https://doi.org/10.1208/s12249-021-02139-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02139-y