Abstract
Therapeutic efficacy of antineoplastic agents possessing a selective target to the nucleus of the cancer cells could be enhanced through novel formulation approaches. Thus, towards improvement of anticancer potential of icariin (ICA) on pancreatic cancer, the drug was entrapped into the polymeric poly lactic-co-glycolic acid (PLGA) with polyethylene glycol (PEG) as diblock copolymer. Optimization of the formulation was done using Statgraphics software to standardize percentages of PEG-PLGA and tween 80 (TW80) to obtain the smallest particle size. The optimized formulation was found to be in nanometer size and low PDI (0.321). Optimized formula enhanced cytotoxicity and apoptotic potential, compared with ICA-raw, against pancreatic cancer cell lines (aspc-1). The entrapment efficiency of the polymeric micelles was 72.34 ± 2.3% with 93.1 ± 6.5% release of ICA within 72 h. There was a twofold increase in apoptosis and sevenfold increase in necrosis of aspc-1 cells when incubated with raw ICA compared to control cells. Further, loss of mitochondrial membrane potential (⁓50-fold) by the ICA-loaded PMs and free drug compared to control cells was found to be due to the generation of ROS. Findings of cell cycle analysis revealed the significant arrest of G2-M phase of aspc-1 cells when incubated with the optimized formulation. Simultaneously, a significantly increased number of cells in pre-G1 revealed maximum apoptotic potential of the drug when delivered via micellar formulation. Finally, upregulation of caspase-3 established the superiority of the PMs approach against pancreatic cancer. In summary, the acquired results highlighted the potentiality of PMs delivery tool for controlling the growth of pancreatic cancer cells for improved efficacy.







Similar content being viewed by others
Change history
19 July 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1208/s12249-023-02614-8
References
Pancreatic cancer (PC) is the seventh leading cause... - Google Scholar [Internet]. [cited 2021 Apr 7]. Available from: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Pancreatic+cancer+%28PC%29+is+the+seventh+leading+cause+of+cancer-related+deaths+worldwide+with+432%2C242+related+deaths+in+2018+&btnG=.
Morgell A, Reisz JA, Ateeb Z, Davanian H, Reinsbach SE, Halimi A, et al. Metabolic characterization of plasma and cyst fluid from cystic precursors to pancreatic cancer patients reveal metabolic signatures of bacterial infection Running title: Pancreatic cysts and bacterial metabolites. medrxiv.org [Internet]. [cited 2021 Apr 7]; Available from: https://doi.org/10.1101/2020.11.03.20225524
Zhan M, Zheng H, Yang Y, He Z, … TX-CM and, 2020 undefined. Cost-effectiveness analysis of maintenance olaparib in patients with metastatic pancreatic cancer and a germline BRCA1/2 mutation based on the POLO. ncbi.nlm.nih.gov [Internet]. [cited 2021 Apr 7]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7751318/
Arici M, Abudayyak M, Boran T, Chemosphere GÖ-, 2020 undefined. Does pendimethalin develop in pancreatic cancer induced inflammation? Elsevier [Internet]. [cited 2021 Apr 7]; Available from: https://www.sciencedirect.com/science/article/pii/S0045653520308377?casa_token=FCNVWYV-OEkAAAAA:Yrnwe3IBO9EcoEKTPPAW3EHnKArhNv-i-8JHKPvXMhEfQ6hGo9CwzcF6MV7HufKWhmCoclN9I0o.
Luo W, Tao J, Zheng L, Cancer TZ-CJ of, 2020 undefined. Current epidemiology of pancreatic cancer: challenges and opportunities. ncbi.nlm.nih.gov [Internet]. [cited 2021 Apr 7]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7797236/.
Sarwar A, Wang B, Su Q, Pharmacology YZ-B, 2020 undefined. MiRNAs directly targeting the key intermediates of biological pathways in pancreatic cancer. Elsevier [Internet]. [cited 2021 Apr 7]; Available from: https://www.sciencedirect.com/science/article/pii/S0006295220305931?casa_token=fBfSqoSqvJsAAAAA:RfOqvBSfo60fUg_7Wg_lykCeBUhHW5jcEzr2NcBv7TqURyo1A3WneBrOzX0tyAp_rIn903ASDWo.
Lan B, Zeng S, Grützmann R, Pilarsky C. Molecular sciences the role of exosomes in pancreatic cancer. mdpi.com [Internet]. [cited 2021 Apr 7]; Available from: www.mdpi.com/journal/ijms.
Liu J, Yu L, Medicine WD-, 2019 undefined. Efficacy and safety of Kanglaite injection combined with radiochemotherapy in the treatment of advanced pancreatic cancer: a PRISMA-compliant meta. ncbi.nlm.nih.gov [Internet]. [cited 2021 Apr 7]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6709199/.
Goodarzi E, Dehkordi AH, Beiranvand R, Naemi H, Khazaei Z. Send Orders for Reprints to reprints@benthamscience.net Epidemiology of the incidence and mortality of pancreas cancer and its relationship with the human development index (HDI) in the world: an ecological study in 2018. ingentaconnect.com [Internet]. 2020 [cited 2021 Apr 7]; Available from: https://www.ingentaconnect.com/content/ben/cpd/pre-prints/content-32660397.
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. American Association for Cancer Research Inc.; 2014. p. 2913–21.
Alhakamy NA, Fahmy UA, Badr-Eldin SM, Ahmed OAA, Asfour HZ, Aldawsari HM, et al. Optimized icariin phytosomes exhibit enhanced cytotoxicity and apoptosis-inducing activities in ovarian cancer cells. Pharmaceutics [Internet]. MDPI AG; 2020 [cited 2020 Dec 10];12. Available from: https://pubmed.ncbi.nlm.nih.gov/32290412/.
Li J, Jiang K, Zhao F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol Rep [Internet]. Spandidos Publications; 2015 [cited 2020 Dec 10];33:2829–36. Available from: http://www.spandidos-publications.com/10.3892/or.2015.3891/abstract
Alhakamy NA, A. Fahmy U, Badr-Eldin SM, Ahmed OAA, Asfour HZ, Aldawsari HM, et al. Optimized icariin phytosomes exhibit enhanced cytotoxicity and apoptosis-inducing activities in ovarian cancer cells. Pharmaceutics [Internet]. MDPI AG; 2020 [cited 2020 May 2];12:346. Available from: https://www.mdpi.com/1999-4923/12/4/346.
Wang Y, Dong H, Zhu M, Ou Y, Zhang J, Luo H, et al. Icariin exterts negative effects on human gastric cancer cell invasion and migration by vasodilator-stimulated phosphoprotein via Rac1 pathway. Eur J Pharmacol Eur J Pharmacol. 2010;635:40–8.
Wang QS, Wang GF, Zhou J, Gao LN, Cui YL. Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis. Int J Pharm. 2016;515:176–85.
Cheng T, Sheng T, Yi Y, Zhang T, Han H. Metabolism profiles of icariin in rats using ultra-high performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry and in vitro enzymatic study. J Chromatogr B Anal Technol Biomed Life Sci. 2016;1033–1034:353–60.
Zhou L, Wang Q, Zhang H, Li Y, Xie S, Biomolecules MX, et al. YAP Inhibition by nuciferine via AMPK-mediated downregulation of HMGCR sensitizes pancreatic cancer cells to gemcitabine. mdpi.com [Internet]. [cited 2021 Apr 7]; Available from: https://www.mdpi.com/2218-273X/9/10/620.
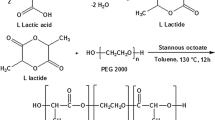
Ahmed OAA, Badr-Eldin SM. Biodegradable self-assembled nanoparticles of PEG-PLGA amphiphilic diblock copolymer as a promising stealth system for augmented vinpocetine brain delivery. Int J Pharm. 2020;588:119778.
Alfaifi MY, Shati AA, Elbehairi SEI, Fahmy UA, Alhakamy NA, Md S. Anti-tumor effect of PEG-coated PLGA nanoparticles of febuxostat on A549 non-small cell lung cancer cells. 3 Biotech. 2020;10.
Alfaifi M, Shati A, Elbehairi S, Biotech UF-3, 2020 undefined. Anti-tumor effect of PEG-coated PLGA nanoparticles of febuxostat on A549 non-small cell lung cancer cells. Springer [Internet]. [cited 2021 Apr 7]; Available from: https://idp.springer.com/authorize/casa?redirect_uri=https://link.springer.com/article/https://doi.org/10.1007/s13205-020-2077-x&casa_token=pB7cz8yUlooAAAAA:WHL0Axsu88KKH_dGA_h6ZJUMLqrESyAtc3OAAUJ4VWl2cegjsmzxGJX1qhV2yIMOjqpO4_D1vPVdzy_Fvw.
Fahmy UA, Aldawsari HM, Badr-Eldin SM, Ahmed OAA, Alhakamy NA, Alsulimani HH, et al. Pharmaceutics the encapsulation of febuxostat into emulsomes strongly enhances the cytotoxic potential of the drug on HCT 116 colon cancer cells. mdpi.com [Internet]. [cited 2021 Apr 7]; Available from: https://gco.iarc.fr/.
Abourehab MAS, Ahmed OAA, Balata GF, Almalki WH. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood–brain barrier. Int J Nanomed. 2018;13:3679–87.
Polat DC, Coskun M. Quantitative determination by HPLC-DAD of icariin, epimedin A, epimedin B, and epimedin C in Epimedium (Berberidaceae) species growing in Turkey. Nat Prod Commun [Internet]. Natural Product Incorporation; 2016 [cited 2021 Apr 7];11:1934578X1601101. Available from: http://journals.sagepub.com/doi/10.1177/1934578X1601101110.
Lagrow AP, Ingham B, Toney MF, Tilley RD. Effect of surfactant concentration and aggregation on the growth kinetics of nickel nanoparticles. J Phys Chem C. 2013;117:16709–18.
Masoudipour E, Kashanian S, Azandaryani AH, Omidfar K, Bazyar E. Surfactant effects on the particle size, zeta potential, and stability of starch nanoparticles and their use in a pH-responsive manner. Cellulose. 2017;24:4217–34.
Koopaei MN, Khoshayand MR, Mostafavi SH, Amini M, Khorramizadeh MR, Tehrani MJ, et al. Docetaxel loaded PEG-PLGA nanoparticles: optimized drug loading, in-vitro cytotoxicity and in-vivo antitumor effect. Iran J Pharm Res. 2014;13:819–34.
Jain A, Jain SK. Formulation and optimization of temozolomide nanoparticles by 3 factor 2 level factorial design. Biomatter. 2013;3.
Sharma N, Madan P, Lin S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: a co-surfactant study. Asian J Pharm Sci. 2015.
Sun Y, Kim J, Kim KT. The effect of steric repulsion between highly branched hydrophilic blocks on inverse cubic mesophase formation in block copolymers. RSC Adv. 2019;9:25423–8.
Moghimi SM, Hunter AC, Andresen TL. Factors controlling nanoparticle pharmacokinetics: an integrated analysis and perspective. Annu Rev Pharmacol Toxicol Ann Rev. 2012;52:481–503.
Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006; 93–102.
Haggag Y, Abdel-Wahab Y, Ojo O, Osman M, El-Gizawy S, El-Tanani M, et al. Preparation and in vivo evaluation of insulin-loaded biodegradable nanoparticles prepared from diblock copolymers of PLGA and PEG. Int J Pharm. 2016;499:236–46.
Zhang K, Tang X, Zhang J, Lu W, Lin X, Zhang Y, et al. PEG-PLGA copolymers: their structure and structure-influenced drug delivery applications. J Control Release 2014;183:77–86. https://doi.org/10.1016/j.jconrel.2014.03.026.
Luo L, Tam J, Maysinger D, Eisenberg A. Cellular internalization of poly(ethylene oxide)-b-poly(epsilon-caprolactone) diblock copolymer micelles. Bioconjug Chem. 2002;13:1259–65.
Md S, Alhakamy NA, Aldawsari HM, Husain M, Kotta S, Abdullah ST, et al. Formulation design, statistical optimization, and in vitro evaluation of a naringenin nanoemulsion to enhance apoptotic activity in A549 lung cancer cells. Pharmaceuticals [Internet]. MDPI AG; 2020 [cited 2020 Dec 17];13:152. Available from: https://www.mdpi.com/1424-8247/13/7/152.
Alfaifi MY, Shati AA, Elbehairi SEI, Fahmy UA, Alhakamy NA, Md S. Anti-tumor effect of PEG-coated PLGA nanoparticles of febuxostat on A549 non-small cell lung cancer cells. 3 Biotech [Internet]. Springer; 2020 [cited 2020 Nov 29];10:133. Available from: https://doi.org/10.1007/s13205-020-2077-x
Boddu SHS, Jwala J, Chowdhury MR, Mitra AK. In vitro evaluation of a targeted and sustained release system for retinoblastoma cells using doxorubicin as a model drug. J Ocul Pharmacol Ther. 2010;26:459–68.
Journal AI, Anari E, Akbarzadeh A, Zarghami N. Artificial cells, nanomedicine, and biotechnology chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line. Taylor Fr [Internet]. Taylor and Francis Ltd.; 2015 [cited 2021 Apr 7];44:1410–6. Available from: https://www.tandfonline.com/action/journalInformation?journalCode=ianb20.
Alhakamy NA, A. Fahmy U, Badr-Eldin SM, Ahmed OAA, Asfour HZ, Aldawsari HM, et al. Optimized icariin phytosomes exhibit enhanced cytotoxicity and apoptosis-inducing activities in ovarian cancer cells. Pharmaceutics [Internet]. MDPI AG; 2020 [cited 2020 Jun 19];12:346. Available from: https://www.mdpi.com/1999-4923/12/4/346.
Alhakamy NA, Ahmed OAA, Fahmy UA, Md S. Development and in vitro evaluation of 2-methoxyestradiol loaded polymeric micelles for enhancing anticancer activities in prostate cancer. Polymers (Basel) [Internet]. MDPI AG; 2021 [cited 2021 Apr 7];13:884. Available from: https://www.mdpi.com/2073-4360/13/6/884.
Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care. 2014;3:511–29.
Murakami H, Kobayashi M, Takeuchi H. Preparation of poly(DL-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. 1999;187:143–52.
Ahmad A, Mishra RK, Vyawahare A, Kumar A, Rehman MU, Qamar W, et al. Thymoquinone (2-isoprpyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: chemistry and biological effects. Saudi Pharm J. 2019; 1113–26.
Ahmad A, Mishra RK, Vyawahare A, Kumar A, Rehman MU, Qamar W, et al. Thymoquinone (2-isoprpyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: chemistry and biological effects. Saudi Pharm J SPJ Off Publ Saudi Pharm Soc [Internet]. Elsevier B.V.; 2019 [cited 2020 Oct 9];27:1113–26. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31885471.
Ahmed OAA, Fahmy UA, Bakhaidar R, El-Moselhy MA, Okbazghi SZ, Ahmed ASF, et al. Omega-3 self-nanoemulsion role in gastroprotection against indomethacin-induced gastric injury in rats. Pharmaceutics. 2020;12.
Alhakamy NA, Ahmed OAA, Aldawsari HM, Alfaifi MY, Eid BG, Abdel-Naim AB, et al. Encapsulation of lovastatin in zein nanoparticles exhibits enhanced apoptotic activity in HepG2 cells. Int J Mol Sci Artic [Internet]. 2019 [cited 2020 May 1]; Available from: www.mdpi.com/journal/ijms.
Alhakamy NA, Ahmed OAA, Aldawsari HM, Alfaifi MY, Eid BG, Abdel-Naim AB, et al. Encapsulation of lovastatin in zein nanoparticles exhibits enhanced apoptotic activity in HepG2 cells. Int J Mol Sci Artic [Internet]. 2019 [cited 2020 Aug 8]; Available from: www.mdpi.com/journal/ijms.
Hosny KM, Bahmdan RH, Alhakamy NA, Alfaleh MA, Ahmed OA, Elkomy MH. Physically optimized nano-lipid carriers augment raloxifene and vitamin D oral bioavailability in healthy humans for management of osteoporosis. J Pharm Sci. 2020.
Acknowledgements
I would like to acknowledge with thanks Dr. Serag Eldin Albhairy, King Khalid University, Abha, Saudi Arabia, and Dr. Essam Rashwan, Vacsera, Cairo, Egypt, for their help and support during the work in the cell line part.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. RG-1-166-42. The author, therefore, acknowledges with thanks DSR for technical and financial support.
Author information
Authors and Affiliations
Contributions
Nabil A. Alhakamy is responsible for the conceptualization, methodology, formal analysis, data curation, writing—original draft preparation, writing—review, editing, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no conflict of interest. The funders/company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1208/s12249-023-02614-8
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alhakamy, N.A. RETRACTED ARTICLE: Development and Evaluation of Icariin-Loaded PLGA-PEG Nanoparticles for Potentiation the Proapoptotic Activity in Pancreatic Cancer Cells. AAPS PharmSciTech 22, 252 (2021). https://doi.org/10.1208/s12249-021-02111-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02111-w