Abstract
Hot-melt extrusion has found extensive application as a feasible pharmaceutical technological option over recent years. HME applications include solubility enhancement, taste masking, and sustained drug release. As bioavailability enhancement is a hot topic of today’s science, one of the main applications of HME is centered on amorphous solid dispersions. This review describes the most significant aspects of HME technology and its use to prepare solid dispersions as a drug formulation strategy to enhance the solubility of poorly soluble drugs. It also addresses molecular and thermodynamic features critical for the physicochemical properties of these systems, mainly in what concerns miscibility and physical stability. Moreover, the importance of applying the Quality by Design philosophy in drug development is also discussed, as well as process analytical technologies in pharmaceutical HME monitoring, under the current standards of product development and regulatory guidance.

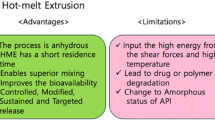
Graphical Abstract





Similar content being viewed by others
Abbreviations
- AG:
-
Adam–Gibbs
- ASD:
-
Amorphous solid dispersions
- BA:
-
Bioavailability
- BCS:
-
Biopharmaceutical Classification Systems
- CPP:
-
Critical process parameter
- CQA:
-
Critical Quality Attribute
- DoE:
-
Design of Experiments
- DSC:
-
Differential Calorimetric Screening
- FDA:
-
Food and Drug Administration USA
- Tg :
-
Glass transition temperature
- HME:
-
Hot-melt extrusion
- HPC:
-
Hydroxypropyl cellulose
- HPMC:
-
Hydroxypropyl methylcellulose (hypromellose)
- HPMCAS:
-
Hypromellose acetate succinate
- ICH:
-
International Conference on Harmonization
- KWW:
-
Kohlrausch–Williams–Watts
- MPD:
-
Melting point depression
- Tm :
-
Melting temperature (Tm)
- PATs:
-
Process Analytical Technologies
- PEG:
-
Polyethylene glycol
- PVP:
-
Poly(vinylpyrrolidone)
- QbD:
-
Quality by Design
References
Lakshman JP. Formulation, Bioavailability, and manufacturing process enhancement: novel applications of melt extrusion in enabling product development. In: Repka MA, Langley N, Di Nunzio J, editors. Melt extrusion: materials, technology and drug product design: Springer; 2013.
Verma S, Rudraraju VS. A systematic approach to design and prepare solid dispersions of poorly water-soluble drug. AAPS PharmSciTech. 2014;15(3):641–57. https://doi.org/10.1208/s12249-014-0093-z.
Pina MF, Zhao M, Pinto JF, Sousa JJ, Craig DQ. The influence of drug physical state on the dissolution enhancement of solid dispersions prepared via hot-melt extrusion: a case study using olanzapine. J Pharm Sci. 2014;103(4):1214–23. https://doi.org/10.1002/jps.23894.
Shah S, Maddineni S, Lu J, Repka MA. Melt extrusion with poorly soluble drugs. Int J Pharm. 2013;453(1):233–52. https://doi.org/10.1016/j.ijpharm.2012.11.001.
Becker K, Salar-Behzadi S, Zimmer A. Solvent-free melting techniques for the preparation of lipid-based solid oral formulations. Pharm Res. 2015;32(5):1519–45. https://doi.org/10.1007/s11095-015-1661-y.
Gao P, Shi Y. Characterization of supersaturatable formulations for improved absorption of poorly soluble drugs. AAPS J. 2012;14(4):703–13. https://doi.org/10.1208/s12248-012-9389-7.
Sarode AL, Sandhu H, Shah N, Malick W, Zia H. Hot melt extrusion for amorphous solid dispersions: temperature and moisture activated drug-polymer interactions for enhanced stability. Mol Pharm. 2013;10(10):3665–75. https://doi.org/10.1021/mp400165b.
Lu M, Guo Z, Li Y, Pang H, Lin L, Liu X, et al. Application of hot melt extrusion for poorly water-soluble drugs: limitations, advances and future prospects. Curr Pharm Des. 2014;20(3):369–87. https://doi.org/10.2174/13816128113199990402.
Repka MA, Shah S, Lu J, Maddineni S, Morott J, Patwardhan K, et al. Melt extrusion: process to product. Expert Opin Drug Deliv. 2012;9(1):105–25. https://doi.org/10.1517/17425247.2012.642365.
Wilson M, Williams MA, Jones DS, Andrews GP. Hot-melt extrusion technology and pharmaceutical application. Ther Deliv. 2012;3(6):787–97. https://doi.org/10.4155/tde.12.26.
Patil H, Tiwari RV, Repka MA. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. 2016;17(1):20–42. https://doi.org/10.1208/s12249-015-0360-7.
Maniruzzaman M, Boateng JS, Snowden MJ, Douroumis D. A review of hot-melt extrusion: process technology to pharmaceutical products. ISRN Pharm. 2012;2012:436763–9. https://doi.org/10.5402/2012/436763.
Chivate A, Garkal AD, Dhas NL, Mehta DTA. Hot melt extrusion: an emerging technique for solubility enhancement of poorly water soluble drugs. PDA J Pharm Sci Technol. 2021:pdajpst.2019.011403. https://doi.org/10.5731/pdajpst.2019.011403.
Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909–26. https://doi.org/10.1080/03639040701498759.
Stankovic M, Frijlink HW, Hinrichs WL. Polymeric formulations for drug release prepared by hot melt extrusion: application and characterization. Drug Discov Today. 2015;20(7):812–23. https://doi.org/10.1016/j.drudis.2015.01.012.
ICH. Q8 (R2) Pharmaceutical Development. August 2009.
ICH. Q9 Quality Risk Management. November 2005.
ICH. Q10 Pharmaceutical Quality System. June 2008.
ICH. Q11 Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities). May 2012.
FDA. Guidance for industry PAT—a framework for innovative pharmaceutical manufacturing and quality assurance. September 2004.
Maniruzzaman M, Nokhodchi A. Continuous manufacturing via hot-melt extrusion and scale up: regulatory matters. Drug Discov Today. 2017;22(2):340–51. https://doi.org/10.1016/j.drudis.2016.11.007.
Kallakunta VR, Sarabu S, Bandari S, Tiwari R, Patil H, Repka MA. An update on the contribution of hot-melt extrusion technology to novel drug delivery in the twenty-first century: part I. Expert Opin Drug Deliv. 2019;16(5):539–50. https://doi.org/10.1080/17425247.2019.1609448.
Hitzer P, Bauerle T, Drieschner T, Ostertag E, Paulsen K, van Lishaut H, et al. Process analytical techniques for hot-melt extrusion and their application to amorphous solid dispersions. Anal Bioanal Chem. 2017;409(18):4321–33. https://doi.org/10.1007/s00216-017-0292-z.
Wesholowski J, Prill S, Berghaus A, Thommes M. Inline UV/Vis spectroscopy as PAT tool for hot-melt extrusion. Drug Deliv Transl Res. 2018;8(6):1595–603. https://doi.org/10.1007/s13346-017-0465-5.
Kelly AL, Gough T, Isreb M, Dhumal R, Jones JW, Nicholson S, et al. In-process rheometry as a PAT tool for hot melt extrusion. Drug Dev Ind Pharm. 2017;44:1–7. https://doi.org/10.1080/03639045.2017.1408641.
Repka MA, Bandari S, Kallakunta VR, Vo AQ, McFall H, Pimparade MB, et al. Melt extrusion with poorly soluble drugs—an integrated review. Int J Pharm. 2018;535(1-2):68–85. https://doi.org/10.1016/j.ijpharm.2017.10.056.
Gryczke A. Hot-melt extrusion process design using process analytical technology. In: Repka AM, Langley N, DiNunzio J, editors. Melt extrusion: materials, technology and drug product design. New York: Springer New York; 2013. p. 397–431.
Netchacovitch L, Thiry J, De Bleye C, Chavez PF, Krier F, Sacré PY, et al. Vibrational spectroscopy and microspectroscopy analyzing qualitatively and quantitatively pharmaceutical hot melt extrudates. J Pharm Biomed Anal. 2015;113:21–33. https://doi.org/10.1016/j.jpba.2015.01.051.
Huang S, O'Donnell KP, Delpon de Vaux SM, O'Brien J, Stutzman J, Williams RO 3rd. Processing thermally labile drugs by hot-melt extrusion: the lesson with gliclazide. Eur J Pharm Biopharm. 2017;119:56–67. https://doi.org/10.1016/j.ejpb.2017.05.014.
Chavan RB, Rathi S, Jyothi V, Shastri NR. Cellulose based polymers in development of amorphous solid dispersions. Asian J Pharm Sci. 2019;14(3):248–64. https://doi.org/10.1016/j.ajps.2018.09.003.
Sangshetti JN, Deshpande M, Zaheer Z, Shinde DB, Arote R. Quality by design approach: regulatory need. Arab J Chem. 2017;10:S3412–S25. https://doi.org/10.1016/j.arabjc.2014.01.025.
Mishra V, Thakur S, Patil A, Shukla A. Quality by design (QbD) approaches in current pharmaceutical set-up. Expert Opin Drug Deliv. 2018;15(8):737–58. https://doi.org/10.1080/17425247.2018.1504768.
Penumetcha SS, Gutta LN, Dhanala H, Yamili S, Challa S, Rudraraju S, et al. Hot melt extruded Aprepitant-Soluplus solid dispersion: preformulation considerations, stability and in vitro study. Drug Dev Ind Pharm. 2016;42(10):1609–20. https://doi.org/10.3109/03639045.2016.1160105.
Aho J, Edinger M, Botker J, Baldursdottir S, Rantanen J. Oscillatory shear rheology in examining the drug-polymer interactions relevant in hot melt extrusion. J Pharm Sci. 2016;105(1):160–7. https://doi.org/10.1016/j.xphs.2015.11.029.
Li S, Tian Y, Jones DS, Andrews GP. Optimising drug solubilisation in amorphous polymer dispersions: rational selection of hot-melt extrusion processing parameters. AAPS PharmSciTech. 2016;17(1):200–13. https://doi.org/10.1208/s12249-015-0450-6.
Chan SY, Qi S, Craig DQ. An investigation into the influence of drug-polymer interactions on the miscibility, processability and structure of polyvinylpyrrolidone-based hot melt extrusion formulations. Int J Pharm. 2015;496(1):95–106. https://doi.org/10.1016/j.ijpharm.2015.09.063.
Simoes MF, Pinto RMA, Simoes S. Hot-melt extrusion in the pharmaceutical industry: toward filing a new drug application. Drug Discov Today. 2019;24(9):1749–68. https://doi.org/10.1016/j.drudis.2019.05.013.
Gupta A, Khan M. Hot-melt extrusion: an FDA perspective on product and process understanding. In: Douroumis D, editor. Hot-melt extrusion: pharmaceutical applications: John Wiley & Sons, Ltd; 2012. p. 323-31.
Chaves LL, Vieira AC, Reis S, Sarmento B, Ferreira DC. Quality by design: discussing and assessing the solid dispersions risk. Curr Drug Deliv. 2014;11(2):253–69. https://doi.org/10.2174/1567201811666140211110943.
Pawar J, Suryawanshi D, Moravkar K, Aware R, Shetty V, Maniruzzaman M, et al. Study the influence of formulation process parameters on solubility and dissolution enhancement of efavirenz solid solutions prepared by hot-melt extrusion: a QbD methodology. Drug Deliv Transl Res. 2018;8(6):1644–57. https://doi.org/10.1007/s13346-018-0481-0.
Desai PM, Hogan RC, Brancazio D, Puri V, Jensen KD, Chun JH, et al. Integrated hot-melt extrusion–injection molding continuous tablet manufacturing platform: effects of critical process parameters and formulation attributes on product robustness and dimensional stability. Int J Pharm. 2017;531(1):332–42. https://doi.org/10.1016/j.ijpharm.2017.08.097.
Patwardhan K, Asgarzadeh F, Dassinger T, Albers J, Repka MA. A quality by design approach to understand formulation and process variability in pharmaceutical melt extrusion processes. J Pharm Pharmacol. 2015;67(5):673–84. https://doi.org/10.1111/jphp.12370.
Zhang L, Mao S. Application of quality by design in the current drug development. Asian J Pharm Sci. 2017;12(1):1–8. https://doi.org/10.1016/j.ajps.2016.07.006.
Markarian J. Defining design space in hot-melt extrusion. Pharm Technol; 2012 [cited 2019 2 Ago]; Available from: http://www.pharmtech.com/defining-design-space-hot-melt-extrusion.
Butreddy A, Bandari S, Repka MA. Quality-by-design in hot melt extrusion based amorphous solid dispersions: an industrial perspective on product development. Eur J Pharm Sci. 2021;158:105655. https://doi.org/10.1016/j.ejps.2020.105655.
Islam MT, Maniruzzaman M, Halsey SA, Chowdhry BZ, Douroumis D. Development of sustained-release formulations processed by hot-melt extrusion by using a quality-by-design approach. Drug Deliv Transl Res. 2014;4(4):377–87. https://doi.org/10.1007/s13346-014-0197-8.
Thiry J, Krier F, Evrard B. A review of pharmaceutical extrusion: critical process parameters and scaling-up. Int J Pharm. 2015;479(1):227–40. https://doi.org/10.1016/j.ijpharm.2014.12.036.
Wu JX, van den Berg F, Sogaard SV, Rantanen J. Fast-track to a solid dispersion formulation using multi-way analysis of complex interactions. J Pharm Sci. 2013;102(3):904–14. https://doi.org/10.1002/jps.23409.
Pawar J, Tayade A, Gangurde A, Moravkar K, Amin P. Solubility and dissolution enhancement of efavirenz hot melt extruded amorphous solid dispersions using combination of polymeric blends: a QbD approach. Eur J Pharm Sci. 2016;88:37–49. https://doi.org/10.1016/j.ejps.2016.04.001.
Lang B, McGinity JW, Williams RO 3rd. Dissolution enhancement of itraconazole by hot-melt extrusion alone and the combination of hot-melt extrusion and rapid freezing—effect of formulation and processing variables. Mol Pharm. 2014;11(1):186–96. https://doi.org/10.1021/mp4003706.
Saerens L, Ghanam D, Raemdonck C, Francois K, Manz J, Kruger R, et al. In-line solid state prediction during pharmaceutical hot-melt extrusion in a 12 mm twin screw extruder using Raman spectroscopy. Eur J Pharm Biopharm. 2014;87(3):606–15. https://doi.org/10.1016/j.ejpb.2014.03.002.
Reitz E, Vervaet C, Neubert RH, Thommes M. Solid crystal suspensions containing griseofulvin—preparation and bioavailability testing. Eur J Pharm Biopharm. 2013;83(2):193–202. https://doi.org/10.1016/j.ejpb.2012.09.012.
Baronsky-Probst J, Moltgen CV, Kessler W, Kessler RW. Process design and control of a twin screw hot melt extrusion for continuous pharmaceutical tamper-resistant tablet production. Eur J Pharm Sci. 2016;87:14–21. https://doi.org/10.1016/j.ejps.2015.09.010.
Chen M, Lu J, Deng W, Singh A, Mohammed NN, Repka MA, et al. Influence of processing parameters and formulation factors on the bioadhesive, temperature stability and drug release properties of hot-melt extruded films containing miconazole. AAPS PharmSciTech. 2014;15(3):522–9. https://doi.org/10.1208/s12249-013-0029-z.
Lang B, McGinity JW, Williams RO 3rd. Hot-melt extrusion—basic principles and pharmaceutical applications. Drug Dev Ind Pharm. 2014;40(9):1133–55. https://doi.org/10.3109/03639045.2013.838577.
Crowley MM, Zhang F, Koleng JJ, McGinity JW. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials. 2002;23(21):4241–8. https://doi.org/10.1016/s0142-9612(02)00187-4.
Gallet G, Carroccio S, Rizzarelli P, Karlsson S. Thermal degradation of poly(ethylene oxide–propylene oxide–ethylene oxide) triblock copolymer: comparative study by SEC/NMR, SEC/MALDI-TOF-MS and SPME/GC-MS. Polymer. 2002;43(4):1081–94. https://doi.org/10.1016/s0032-3861(01)00677-2.
Watanabe T, Okabayashi M, Kurokawa D, Nishimoto Y, Ozawa T, Kawasaki H, et al. Determination of primary bond scissions by mass spectrometric analysis of ultrasonic degradation products of poly(ethylene oxide-block-propylene oxide) copolymers. J Mass Spectrom. 2010;45(7):799–805. https://doi.org/10.1002/jms.1771.
Shojaee S, Cumming I, Kaialy W, Nokhodchi A. The influence of vitamin E succinate on the stability of polyethylene oxide PEO controlled release matrix tablets. Colloids Surf B: Biointerfaces. 2013;111:486–92. https://doi.org/10.1016/j.colsurfb.2013.06.038.
Carrasco F, Pagès P, Gámez-Pérez J, Santana OO, Maspoch ML. Processing of poly(lactic acid): characterization of chemical structure, thermal stability and mechanical properties. Polym Degrad Stab. 2010;95(2):116–25. https://doi.org/10.1016/j.polymdegradstab.2009.11.045.
Zhang T, Zhou S, Gao X, Yang Z, Sun L, Zhang D. A multi-scale method for modeling degradation of bioresorbable polyesters. Acta Biomater. 2017;50:462–75. https://doi.org/10.1016/j.actbio.2016.12.046.
Liu W-C, Halley PJ, Gilbert RG. Mechanism of degradation of starch, a highly branched polymer, during extrusion. Macromolecules. 2010;43(6):2855–64. https://doi.org/10.1021/ma100067x.
Hughey JR, Keen JM, Miller DA, Brough C, McGinity JW. Preparation and characterization of fusion processed solid dispersions containing a viscous thermally labile polymeric carrier. Int J Pharm. 2012;438(1-2):11–9. https://doi.org/10.1016/j.ijpharm.2012.08.032.
Lu G, Kalyon DM, Yilgör I, Yilgör E. Rheology and extrusion of medical-grade thermoplastic polyurethane. Polym Eng Sci. 2003;43(12):1863–77. https://doi.org/10.1002/pen.10158.
Dong Z, Choi DS. Hydroxypropyl methylcellulose acetate succinate: potential drug-excipient incompatibility. AAPS PharmSciTech. 2008;9(3):991–7. https://doi.org/10.1208/s12249-008-9138-5.
Alexy P, Lacı́k I, Šimková B, Bakoš D, Na P, Liptaj T, et al. Effect of melt processing on thermo-mechanical degradation of poly(vinyl alcohol)s. Polym Degrad Stab. 2004;85(2):823–30. https://doi.org/10.1016/j.polymdegradstab.2004.02.011.
Lin S-Y, Yu H-L, Li M-J. Formation of six-membered cyclic anhydrides by thermally induced intramolecular ester condensation in Eudragit E film. Polymer. 1999;40(12):3589–93. https://doi.org/10.1016/s0032-3861(98)00488-1.
Lin S-Y, Yu H-L. Thermal stability of methacrylic acid copolymers of Eudragits L, S, and L30D and the acrylic acid polymer of carbopol. J Polym Sci A Polym Chem. 1999;37(13):2061–7. https://doi.org/10.1002/(sici)1099-0518(19990701).
Hughey JR, DiNunzio JC, Bennett RC, Brough C, Miller DA, Ma H, et al. Dissolution enhancement of a drug exhibiting thermal and acidic decomposition characteristics by fusion processing: a comparative study of hot melt extrusion and KinetiSol dispersing. AAPS PharmSciTech. 2010;11(2):760–74. https://doi.org/10.1208/s12249-010-9431-y.
Stroyer A, McGinity JW, Leopold CS. Solid state interactions between the proton pump inhibitor omeprazole and various enteric coating polymers. J Pharm Sci. 2006;95(6):1342–53. https://doi.org/10.1002/jps.20450.
Matić J, Paudel A, Bauer H, Garcia RAL, Biedrzycka K, Khinast JG. Developing HME-based drug products using emerging science: a fast-track roadmap from concept to clinical batch. AAPS PharmSciTech. 2020;21(5):176. https://doi.org/10.1208/s12249-020-01713-0.
Jelić D. Thermal stability of amorphous solid dispersions. Molecules. 2021;26(1):238. https://doi.org/10.3390/molecules26010238.
Lin X, Hu Y, Liu L, Su L, Li N, Yu J, et al. Physical stability of amorphous solid dispersions: a physicochemical perspective with thermodynamic, kinetic and environmental aspects. Pharm Res. 2018;35(6):125. https://doi.org/10.1007/s11095-018-2408-3.
Pandi P, Bulusu R, Kommineni N, Khan W, Singh M. Amorphous solid dispersions: an update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int J Pharm. 2020;586:119560. https://doi.org/10.1016/j.ijpharm.2020.119560.
Hancock BC, Shamblin SL, Zografi G. Molecular mobility of amorphous pharmaceutical solids below their glass transition temperatures. Pharm Res. 1995;12(6):799–806. https://doi.org/10.1023/a:1016292416526.
Laitinen R, Lobmann K, Strachan CJ, Grohganz H, Rades T. Emerging trends in the stabilization of amorphous drugs. Int J Pharm. 2013;453(1):65–79. https://doi.org/10.1016/j.ijpharm.2012.04.066.
Baghel S, Cathcart H, O'Reilly NJ. Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J Pharm Sci. 2016;105(9):2527–44. https://doi.org/10.1016/j.xphs.2015.10.008.
Janssens S, Van den Mooter G. Review: physical chemistry of solid dispersions. J Pharm Pharmacol. 2009;61(12):1571–86. https://doi.org/10.1211/jpp/61.12.0001.
Zhu DA, Zografi G, Gao P, Gong Y, Zhang GGZ. Modeling physical stability of amorphous solids based on temperature and moisture stresses. J Pharm Sci. 2016;105(9):2932–9. https://doi.org/10.1016/j.xphs.2016.03.029.
Bhardwaj SP, Arora KK, Kwong E, Templeton A, Clas SD, Suryanarayanan R. Correlation between molecular mobility and physical stability of amorphous itraconazole. Mol Pharm. 2013;10(2):694–700. https://doi.org/10.1021/mp300487u.
Miyanishi H, Nemoto T, Mizuno M, Mimura H, Kitamura S, Iwao Y, et al. Evaluation of crystallization behavior on the surface of nifedipine solid dispersion powder using inverse gas chromatography. Pharm Res. 2013;30(2):502–11. https://doi.org/10.1007/s11095-012-0896-0.
Shah N, Sandhu H, Choi DS, Chokshi H, Malick AW. Amorphous solid dispersions: theory and practice. New York: Springer; 2014.
Gupta SS, Solanki N, Serajuddin AT. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion, IV: Affinisol HPMC HME polymers. AAPS PharmSciTech. 2016;17(1):148–57. https://doi.org/10.1208/s12249-015-0426-6.
Mao C, Chamarthy SP, Pinal R. Time-dependence of molecular mobility during structural relaxation and its impact on organic amorphous solids: an investigation based on a calorimetric approach. Pharm Res. 2006;23(8):1906–17. https://doi.org/10.1007/s11095-006-9008-3.
Surana R, Pyne A, Rani M, Suryanarayanan R. Measurement of enthalpic relaxation by differential scanning calorimetry—effect of experimental conditions. Thermochim Acta. 2005;433(1-2):173–82. https://doi.org/10.1016/j.tca.2005.02.014.
Hasegawa S, Ke P, Buckton G. Determination of the structural relaxation at the surface of amorphous solid dispersion using inverse gas chromatography. J Pharm Sci. 2009;98(6):2133–9. https://doi.org/10.1002/jps.21573.
Bansal SS, Kaushal AM, Bansal AK. Enthalpy relaxation studies of two structurally related amorphous drugs and their binary dispersions. Drug Dev Ind Pharm. 2010;36(11):1271–80. https://doi.org/10.3109/03639041003753847.
Kakumanu VK, Bansal AK. Enthalpy relaxation studies of celecoxib amorphous mixtures. Pharm Res. 2002;19(12):1873–8. https://doi.org/10.1023/a:1021453810624.
Mao C, Prasanth Chamarthy S, Byrn SR, Pinal R. A calorimetric method to estimate molecular mobility of amorphous solids at relatively low temperatures. Pharm Res. 2006;23(10):2269–76. https://doi.org/10.1007/s11095-006-9071-9.
Karmwar P, Graeser K, Gordon KC, Strachan CJ, Rades T. Investigation of properties and recrystallisation behaviour of amorphous indomethacin samples prepared by different methods. Int J Pharm. 2011;417(1-2):94–100. https://doi.org/10.1016/j.ijpharm.2010.12.019.
Mao C, Chamarthy SP, Pinal R. Calorimetric study and modeling of molecular mobility in amorphous organic pharmaceutical compounds using a modified Adam-Gibbs approach. J Phys Chem B. 2007;111(46):13243–52. https://doi.org/10.1021/jp072577.
Aso Y, Yoshioka S, Kojima S. Molecular mobility-based estimation of the crystallization rates of amorphous nifedipine and phenobarbital in poly(vinylpyrrolidone) solid dispersions. J Pharm Sci. 2004;93(2):384–91. https://doi.org/10.1002/jps.10526.
Berthier L, Coslovich D. Novel approach to numerical measurements of the configurational entropy in supercooled liquids. Proc Natl Acad Sci U S A. 2014;111(32):11668–72. https://doi.org/10.1073/pnas.1407934111.
Qian F, Huang J, Hussain MA. Drug-polymer solubility and miscibility: stability consideration and practical challenges in amorphous solid dispersion development. J Pharm Sci. 2010;99(7):2941–7. https://doi.org/10.1002/jps.22074.
Forster A, Hempenstall J, Tucker, Rades T. The potential of small-scale fusion experiments and the Gordon-Taylor equation to predict the suitability of drug/polymer blends for melt extrusion. Drug Dev Ind Pharm. 2001;27(6):549–60. https://doi.org/10.1081/ddc-100105180.
Nair R, Nyamweya N, Gönen S, Martı́nez-Miranda LJ, Hoag SW. Influence of various drugs on the glass transition temperature of poly(vinylpyrrolidone): a thermodynamic and spectroscopic investigation. Int J Pharm. 2001;225(1-2):83–96. https://doi.org/10.1016/s0378-5173(01)00767-0.
Jensen KT, Larsen FH, Lobmann K, Rades T, Grohganz H. Influence of variation in molar ratio on co-amorphous drug-amino acid systems. Eur J Pharm Biopharm. 2016;107:32–9. https://doi.org/10.1016/j.ejpb.2016.06.020.
Rask MB, Knopp MM, Olesen NE, Holm R, Rades T. Influence of PVP/VA copolymer composition on drug-polymer solubility. Eur J Pharm Sci. 2016;85:10–7. https://doi.org/10.1016/j.ejps.2016.01.026.
O'Donnell KP, Woodward WH. Dielectric spectroscopy for the determination of the glass transition temperature of pharmaceutical solid dispersions. Drug Dev Ind Pharm. 2015;41(6):959–68. https://doi.org/10.3109/03639045.2014.919314.
Greenhalgh DJ, Williams AC, Timmins P, York P. Solubility parameters as predictors of miscibility in solid dispersions. J Pharm Sci. 1999;88(11):1182–90. https://doi.org/10.1021/js9900856.
Van Krevelen DW, Te Nijenhuis K. Chapter 7—Cohesive properties and solubility. In:Properties of polymers. 4th ed. Amsterdam: Elsevier; 2009. p. 189–227.
Just S, Sievert F, Thommes M, Breitkreutz J. Improved group contribution parameter set for the application of solubility parameters to melt extrusion. Eur J Pharm Biopharm. 2013;85(3 Pt B):1191–9. https://doi.org/10.1016/j.ejpb.2013.04.006.
Forster A, Hempenstall J, Tucker I, Rades T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int J Pharm. 2001;226(1-2):147–61. https://doi.org/10.1016/s0378-5173(01)00801-8.
Baghel S, Cathcart H, O'Reilly NJ. Theoretical and experimental investigation of drug-polymer interaction and miscibility and its impact on drug supersaturation in aqueous medium. Eur J Pharm Biopharm. 2016;107:16–31. https://doi.org/10.1016/j.ejpb.2016.06.024.
Djuris J, Nikolakakis I, Ibric S, Djuric Z, Kachrimanis K. Preparation of carbamazepine-Soluplus solid dispersions by hot-melt extrusion, and prediction of drug-polymer miscibility by thermodynamic model fitting. Eur J Pharm Biopharm. 2013;84(1):228–37. https://doi.org/10.1016/j.ejpb.2012.12.018.
Zhang Y, Luo R, Chen Y, Ke X, Hu D, Han M. Application of carrier and plasticizer to improve the dissolution and bioavailability of poorly water-soluble baicalein by hot melt extrusion. AAPS PharmSciTech. 2014;15(3):560–8. https://doi.org/10.1208/s12249-013-0071-x.
Yoo SU, Krill SL, Wang Z, Telang C. Miscibility/stability considerations in binary solid dispersion systems composed of functional excipients towards the design of multi-component amorphous systems. J Pharm Sci. 2009;98(12):4711–23. https://doi.org/10.1002/jps.21779.
Yang M, Wang P, Gogos C. Prediction of acetaminophen's solubility in poly(ethylene oxide) at room temperature using the Flory-Huggins theory. Drug Dev Ind Pharm. 2013;39(1):102–8. https://doi.org/10.3109/03639045.2012.659188.
Marsac PJ, Shamblin SL, Taylor LS. Theoretical and practical approaches for prediction of drug-polymer miscibility and solubility. Pharm Res. 2006;23(10):2417–26. https://doi.org/10.1007/s11095-006-9063-9.
Marsac PJ, Konno H, Taylor LS. A comparison of the physical stability of amorphous felodipine and nifedipine systems. Pharm Res. 2006;23(10):2306–16. https://doi.org/10.1007/s11095-006-9047-9.
Tian Y, Booth J, Meehan E, Jones DS, Li S, Andrews GP. Construction of drug-polymer thermodynamic phase diagrams using Flory-Huggins interaction theory: identifying the relevance of temperature and drug weight fraction to phase separation within solid dispersions. Mol Pharm. 2013;10(1):236–48. https://doi.org/10.1021/mp300386v.
Shah SM, Jain AS, Kaushik R, Nagarsenker MS, Nerurkar MJ. Preclinical formulations: insight, strategies, and practical considerations. AAPS PharmSciTech. 2014;15(5):1307–23. https://doi.org/10.1208/s12249-014-0156-1.
Thakral S, Thakral NK. Prediction of drug-polymer miscibility through the use of solubility parameter based Flory-Huggins interaction parameter and the experimental validation: PEG as model polymer. J Pharm Sci. 2013;102(7):2254–63. https://doi.org/10.1002/jps.23583.
Hengsawas Surasarang S, Keen JM, Huang S, Zhang F, McGinity JW, Williams RO 3rd. Hot melt extrusion versus spray drying: hot melt extrusion degrades albendazole. Drug Dev Ind Pharm. 2017;43(5):797–811. https://doi.org/10.1080/03639045.2016.1220577.
DiNunzio JC, Martin ZFC, McGinity JW. Melt Extrusion. In: Williams III RO, Watts AB, Miller DA, editors. Formulating poorly water soluble drugs. New York: Springer; 2012. p. 331–62.
Maddineni S, Battu SK, Morott J, Majumdar S, Murthy SN, Repka MA. Influence of process and formulation parameters on dissolution and stability characteristics of Kollidon(R) VA 64 hot-melt extrudates. AAPS PharmSciTech. 2015;16(2):444–54. https://doi.org/10.1208/s12249-014-0226-4.
Mendonsa N, Almutairy B, Kallakunta VR, Sarabu S, Thipsay P, Bandari S, et al. Manufacturing strategies to develop amorphous solid dispersions: an overview. J Drug Deliv Sci Technol. 2020;55:101459. https://doi.org/10.1016/j.jddst.2019.101459.
Dinunzio JC, Brough C, Hughey JR, Miller DA, Williams RO 3rd, McGinity JW. Fusion production of solid dispersions containing a heat-sensitive active ingredient by hot melt extrusion and Kinetisol dispersing. Eur J Pharm Biopharm. 2010;74(2):340–51. https://doi.org/10.1016/j.ejpb.2009.09.007.
Yu LX. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25(4):781–91. https://doi.org/10.1007/s11095-007-9511-1.
Elkhabaz A, Sarkar S, Simpson GJ, Taylor LS. Characterization of phase transformations for amorphous solid dispersions of a weakly basic drug upon dissolution in biorelevant media. Pharm Res. 2019;36(12):174. https://doi.org/10.1007/s11095-019-2718-0.
Elkhabaz A, Sarkar S, Dinh JK, Simpson GJ, Taylor LS. Variation in supersaturation and phase behavior of ezetimibe amorphous solid dispersions upon dissolution in different biorelevant media. Mol Pharm. 2018;15(1):193–206. https://doi.org/10.1021/acs.molpharmaceut.7b00814.
Ashwathy P, Anto AT, Sudheesh MS. A mechanistic review on the dissolution phase behavior and supersaturation stabilization of amorphous solid dispersions. Drug Dev Ind Pharm. 2021;47(1):1–11. https://doi.org/10.1080/03639045.2021.1879843.
Puppolo MM, Hughey JR, Dillon T, Storey D, Jansen-Varnum S. Biomimetic dissolution: a tool to predict amorphous solid dispersion performance. AAPS PharmSciTech. 2017;18(8):2841–53. https://doi.org/10.1208/s12249-017-0783-4.
Litou C, Turner DB, Holmstock N, Ceulemans J, Box KJ, Kostewicz E, et al. Combining biorelevant in vitro and in silico tools to investigate the in vivo performance of the amorphous solid dispersion formulation of etravirine in the fed state. Eur J Pharm Sci. 2020;149:105297. https://doi.org/10.1016/j.ejps.2020.105297.
Kambayashi A, Kiyota T, Fujiwara M, Dressman JB. PBPK modeling coupled with biorelevant dissolution to forecast the oral performance of amorphous solid dispersion formulations. Eur J Pharm Sci. 2019;135:83–90. https://doi.org/10.1016/j.ejps.2019.05.013.
Boyd BJ, Bergström CAS, Vinarov Z, Kuentz M, Brouwers J, Augustijns P, et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur J Pharm Sci. 2019;137:104967. https://doi.org/10.1016/j.ejps.2019.104967.
Keen JM, McGinity JW, Williams RO 3rd. Enhancing bioavailability through thermal processing. Int J Pharm. 2013;450(1-2):185–96. https://doi.org/10.1016/j.ijpharm.2013.04.042.
Seibert KD, Collins PC, Fisher E. Milling operations in the pharmaceutical industry. In: Ende DJ, editor. Chemical engineering in the pharmaceutical industry. New Jersey: John Wiley & Sons, Inc.; 2010. p. 365–78.
Agrawal A, Dudhedia M, Deng W, Shepard K, Zhong L, Povilaitis E, et al. Development of tablet formulation of amorphous solid dispersions prepared by hot melt extrusion using quality by design approach. AAPS PharmSciTech. 2016;17(1):214–32. https://doi.org/10.1208/s12249-015-0472-0.
Kolter K, Karl M, Gryczke A. Hot-melt extrusion with BASF pharma polymers: extrusion compendium. Germany: BASF SE - Pharma Ingredients and Services; 2012.
Iyer R, Hegde S, Zhang YE, Dinunzio J, Singhal D, Malick A, et al. The impact of hot melt extrusion and spray drying on mechanical properties and tableting indices of materials used in pharmaceutical development. J Pharm Sci. 2013;102(10):3604–13. https://doi.org/10.1002/jps.23661.
Carley JF, McKelvey JM. Extruder scale-up theory and experiments. Ind Eng Chem. 1953;45(5):989–92. https://doi.org/10.1021/ie50521a036.
Nakatani M. Short communication: scale-up theory for twin-screw extruder, keeping the resin temperature unchanged. Adv Polym Technol. 1998;17(1):19–22. https://doi.org/10.1002/(sici)1098-2329(199821).
Chung CI. On the scale-up of plasticating extruder screws. Polym Eng Sci. 1984;24(9):626–32. https://doi.org/10.1002/pen.760240904.
Meijer HEH, Elemans PHM. The modeling of continuous mixers. Part I: The corotating twin-screw extruder. Polym Eng Sci. 1988;28(5):275–90. https://doi.org/10.1002/pen.760280504.
Agur EE. Extruder scale-up in a corotating twin-screw extrusion compounding process. Adv Polym Technol. 1986;6(2):225–31. https://doi.org/10.1002/adv.1986.060060208.
Bigio D, Wang K. Scale-up rules for mixing in a non-intermeshing twin-screw extruder. Polym Eng Sci. 1996;36(23):2832–9. https://doi.org/10.1002/pen.10684.
Hughey J. A practical guide to hot-melt extrusion scale-up for pharmaceutical applications. Pharm Technol. 2014;Solid Dosage & Excipients:25-9.
Ghosh I, Vippagunta R, Li S, Vippagunta S. Key considerations for optimization of formulation and melt-extrusion process parameters for developing thermosensitive compound. Pharm Dev Technol. 2012;17(4):502–10. https://doi.org/10.3109/10837450.2010.550624.
Agrawal AM, Dudhedia MS, Zimny E. Hot melt extrusion: development of an amorphous solid dispersion for an insoluble drug from mini-scale to clinical scale. AAPS PharmSciTech. 2016;17(1):133–47. https://doi.org/10.1208/s12249-015-0425-7.
Bochmann ES, Steffens KE, Gryczke A, Wagner KG. Numerical simulation of hot-melt extrusion processes for amorphous solid dispersions using model-based melt viscosity. Eur J Pharm Biopharm. 2018;124:34–42. https://doi.org/10.1016/j.ejpb.2017.12.001.
Dubey SP, Abhyankar HA, Marchante V, Brighton JL, Blackburn K, Temple C, et al. Modelling and validation of synthesis of poly lactic acid using an alternative energy source through a continuous reactive extrusion process. Polymers. 2016;8(4):164. https://doi.org/10.3390/polym8040164.
Zecevic DE, Evans RC, Paulsen K, Wagner KG. From benchtop to pilot scale-experimental study and computational assessment of a hot-melt extrusion scale-up of a solid dispersion of dipyridamole and copovidone. Int J Pharm. 2018;537(1-2):132–9. https://doi.org/10.1016/j.ijpharm.2017.12.033.
Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5(5):442–53. https://doi.org/10.1016/j.apsb.2015.07.003.
Loreti G, Maroni A, Del Curto MD, Melocchi A, Gazzaniga A, Zema L. Evaluation of hot-melt extrusion technique in the preparation of HPC matrices for prolonged release. Eur J Pharm Sci. 2014;52:77–85. https://doi.org/10.1016/j.ejps.2013.10.014.
Wilson MR, Jones DS, Andrews GP. The development of sustained release drug delivery platforms using melt-extruded cellulose-based polymer blends. J Pharm Pharmacol. 2017;69(1):32–42. https://doi.org/10.1111/jphp.12656.
Yi S, Wang J, Lu Y, Ma R, Gao Q, Liu S, et al. Novel hot melt extruded matrices of hydroxypropyl cellulose and amorphous felodipine-plasticized hydroxypropyl methylcellulose as controlled release systems. AAPS PharmSciTech. 2019;20(6):219. https://doi.org/10.1208/s12249-019-1435-7.
Almutairi M, Almutairy B, Sarabu S, Almotairy A, Ashour E, Bandari S, et al. Processability of AquaSolve LG polymer by hot-melt extrusion: effects of pressurized CO2 on physicomechanical properties and API stability. J Drug Deliv Sci Technol. 2019;52:165–76. https://doi.org/10.1016/j.jddst.2019.04.029.
Xue X, Chen G, Xu X, Wang J, Wang J, Ren L. A combined utilization of Plasdone-S630 and HPMCAS-HF in ziprasidone hydrochloride solid dispersion by hot-melt extrusion to enhance the oral bioavailability and no food effect. AAPS PharmSciTech. 2019;20(1):37. https://doi.org/10.1208/s12249-018-1216-8.
Wu CY, Lui WB, Peng J. Optimization of extrusion variables and maleic anhydride content on biopolymer blends based on poly(hydroxybutyrate-co-hydroxyvalerate)/poly(vinyl acetate) with tapioca starch. Polymers. 2018;10(8):827. https://doi.org/10.3390/polym10080827.
Pimparade MB, Vo A, Maurya AS, Bae J, Morott JT, Feng X, et al. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. Eur J Pharm Biopharm. 2017;119:81–90. https://doi.org/10.1016/j.ejpb.2017.06.004.
Fukuda M, Peppas NA, McGinity JW. Properties of sustained release hot-melt extruded tablets containing chitosan and xanthan gum. Int J Pharm. 2006;310(1-2):90–100. https://doi.org/10.1016/j.ijpharm.2005.11.042.
Verhoeven E, Vervaet C, Remon JP. Xanthan gum to tailor drug release of sustained-release ethylcellulose mini-matrices prepared via hot-melt extrusion: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2006;63(3):320–30. https://doi.org/10.1016/j.ejpb.2005.12.004.
Leelakanok N, Geary SM, Salem AK. Antitumor efficacy and toxicity of 5-fluorouracil-loaded poly(lactide co-glycolide) pellets. J Pharm Sci. 2018;107(2):690–7. https://doi.org/10.1016/j.xphs.2017.10.005.
Bode C, Kranz H, Fivez A, Siepmann F, Siepmann J. Often neglected: PLGA/PLA swelling orchestrates drug release: HME implants. J Control Release. 2019;306:97–107. https://doi.org/10.1016/j.jconrel.2019.05.039.
Kamel R, Abbas H. PLGA-based monolithic filaments prepared by hot-melt extrusion: In-vitro comparative study. Ann Pharm Fr. 2018;76(2):97–106. https://doi.org/10.1016/j.pharma.2017.09.002.
Heller J, Barr J, Ng S, Shen HR, Gurny R, Schwach-Abdelaoui K, et al. Development of poly(ortho esters) and their application for bovine serum albumin and bupivacaine delivery. J Control Release. 2002;78(1-3):133–41. https://doi.org/10.1016/s0168-3659(01)00482-5.
Heller J, Barr J, Ng SY, Shen HR, Schwach-Abdellaoui K, Einmahl S, et al. Poly(ortho esters)—their development and some recent applications. Eur J Pharm Biopharm. 2000;50(1):121–8. https://doi.org/10.1016/s0939-6411(00)00085-0.
Verstraete G, Vandenbussche L, Kasmi S, Nuhn L, Brouckaert D, Van Renterghem J, et al. Thermoplastic polyurethane-based intravaginal rings for prophylaxis and treatment of (recurrent) bacterial vaginosis. Int J Pharm. 2017;529(1-2):218–26. https://doi.org/10.1016/j.ijpharm.2017.06.076.
Verstraete G, Mertens P, Grymonpre W, Van Bockstal PJ, De Beer T, Boone MN, et al. A comparative study between melt granulation/compression and hot melt extrusion/injection molding for the manufacturing of oral sustained release thermoplastic polyurethane matrices. Int J Pharm. 2016;513(1-2):602–11. https://doi.org/10.1016/j.ijpharm.2016.09.072.
Verstraete G, Van Renterghem J, Van Bockstal PJ, Kasmi S, De Geest BG, De Beer T, et al. Hydrophilic thermoplastic polyurethanes for the manufacturing of highly dosed oral sustained release matrices via hot melt extrusion and injection molding. Int J Pharm. 2016;506(1-2):214–21. https://doi.org/10.1016/j.ijpharm.2016.04.057.
Li LC, Deng J, Stephens D. Polyanhydride implant for antibiotic delivery—from the bench to the clinic. Adv Drug Deliv Rev. 2002;54(7):963–86. https://doi.org/10.1016/s0169-409x(02)00053-4.
Deng JS, Meisters M, Li L, Setesak J, Claycomb L, Tian Y, et al. The development of an injection-molding process for a polyanhydride implant containing gentamicin sulfate. PDA J Pharm Sci Technol. 2002;56(2):65–77.
Simões MF, Pereira A, Cardoso S, Cadonau S, Werner K, Pinto RMA, et al. A 5-stage approach for a systematic screening and development of etravirine amorphous solid dispersions by hot-melt extrusion. Mol Pharm. 2019. https://doi.org/10.1021/acs.molpharmaceut.9b00996.
Evans RC, Bochmann ES, Kyeremateng SO, Gryczke A, Wagner KG. Holistic QbD approach for hot-melt extrusion process design space evaluation: linking materials science, experimentation and process modeling. Eur J Pharm Biopharm. 2019;141:149–60. https://doi.org/10.1016/j.ejpb.2019.05.021.
Ma X, Huang S, Lowinger MB, Liu X, Lu X, Su Y, et al. Influence of mechanical and thermal energy on nifedipine amorphous solid dispersions prepared by hot melt extrusion: preparation and physical stability. Int J Pharm. 2019;561:324–34. https://doi.org/10.1016/j.ijpharm.2019.03.014.
Zi P, Zhang C, Ju C, Su Z, Bao Y, Gao J, et al. Solubility and bioavailability enhancement study of lopinavir solid dispersion matrixed with a polymeric surfactant—Soluplus. Eur J Pharm Sci. 2019;134:233–45. https://doi.org/10.1016/j.ejps.2019.04.022.
Simões MF, Nogueira BA, Tabanez AM, Fausto R, Pinto RMA, Simões S. Enhanced solid-state stability of amorphous ibrutinib formulations prepared by hot-melt extrusion. Int J Pharm. 2020;579:119156. https://doi.org/10.1016/j.ijpharm.2020.119156.
Ugaonkar SR, Wesenberg A, Wilk J, Seidor S, Mizenina O, Kizima L, et al. A novel intravaginal ring to prevent HIV-1, HSV-2, HPV, and unintended pregnancy. J Control Release. 2015;213:57–68. https://doi.org/10.1016/j.jconrel.2015.06.018.
Almeida A, Possemiers S, Boone MN, De Beer T, Quinten T, Van Hoorebeke L, et al. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur J Pharm Biopharm. 2011;77(2):297–305. https://doi.org/10.1016/j.ejpb.2010.12.004.
Feng Z, Li M, Wang W. Improvement of dissolution and tabletability of carbamazepine solid dispersions with high drug loading prepared by hot-melt extrusion. Pharmazie. 2019;74(9):523–8. https://doi.org/10.1691/ph.2019.9008.
Meng F, Meckel J, Zhang F. Investigation of itraconazole ternary amorphous solid dispersions based on povidone and Carbopol. Eur J Pharm Sci. 2017;106:413–21. https://doi.org/10.1016/j.ejps.2017.06.019.
Bhagurkar AM, Darji M, Lakhani P, Thipsay P, Bandari S, Repka MA. Effects of formulation composition on the characteristics of mucoadhesive films prepared by hot-melt extrusion technology. J Pharm Pharmacol. 2019;71(3):293–305. https://doi.org/10.1111/jphp.13046.
Palazi E, Karavas E, Barmpalexis P, Kostoglou M, Nanaki S, Christodoulou E, et al. Melt extrusion process for adjusting drug release of poorly water soluble drug felodipine using different polymer matrices. Eur J Pharm Sci. 2018;114:332–45. https://doi.org/10.1016/j.ejps.2018.01.004.
Xu X, Siddiqui A, Srinivasan C, Mohammad A, Rahman Z, Korang-Yeboah M, et al. Evaluation of abuse-deterrent characteristics of tablets prepared via hot-melt extrusion. AAPS PharmSciTech. 2019;20(6):230. https://doi.org/10.1208/s12249-019-1448-2.
Acknowledgements
The authors thank Teresa Filipe for her support on illustrations.
Funding
This work was supported by Bluepharma, Coimbra, Portugal, and by Fundação para a Ciência e Tecnologia (FCT), Portugal (Ph.D. fellowship PD/BDE/135149/2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Simões, M.F., Pinto, R.M.A. & Simões, S. Hot-Melt Extrusion: a Roadmap for Product Development. AAPS PharmSciTech 22, 184 (2021). https://doi.org/10.1208/s12249-021-02017-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02017-7