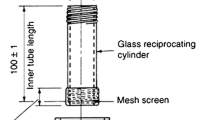
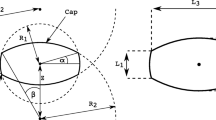
Abstract
Tablets are the most customarily used solid oral unit dosage form for its better patient compliance. Preparation of these tablets include granulation, granule drying, die filling, and tablet coating as few unit operations and evaluation tests like dissolution test and disintegration test. These are the most crucial segments influencing the quality of the tablet. Critical analysis of the impact of factors like flow pattern, temperature, velocity, and other properties of fluid affecting the unit operations is obligatory to enhance their efficiency. Computational fluid dynamics (CFD), a combined mathematical and numerical approach, is used to analyze the process parameters of fluid affecting the abovementioned processes during tablet formulation. The equations governing the laws of conservation of energy, mass, and momentum are solved numerically utilizing CFD software for better understanding of the role of fluids within the tablet processing steps. This review not only focuses on discrete explanations on how CFD is utilized in formulation and evaluation of tablet but it is also a compilation of multiple research works performed on each unit operation by applying CFD.









Similar content being viewed by others
References
Shaw CT. Using Computational Fluid Dynamics, vol. 315. Washington: Prentice Hall; 1992.
Al-Arkawazi S, Marie C, Benhabib K, Coorevits P. Modeling the hydrodynamic forces between fluid–granular medium by coupling DEM–CFD. Chem Eng Res Des [internet]. 2017;117:439–47. Available from:. https://doi.org/10.1016/j.cherd.2016.11.002.
Yang YC, Ouyang Y, Zhang N, Yu QJ, Arowo M. A review on computational fluid dynamic simulation for rotating packed beds. J Chem Technol Biotechnol. 2019;94(4):1017–31.
Sharma C, Malhotra D, Rathore AS. Review of computational fluid dynamics applications in biotechnology processes. Biotechnol Prog. 2011;27(6):1497–510.
Xia B, Sun DW. Applications of computational fluid dynamics (CFD) in the food industry: a review. Comput Electron Agric. 2002;34(1–3):5–24.
Morris PD, Narracott A, Von Tengg-Kobligk H, Soto DAS, Hsiao S, Lungu A, et al. Computational fluid dynamics modelling in cardiovascular medicine. Heart. 2016;102(1):18–28.
Garcia GJM, Bailie N, Martins DA, Kimbell JS. Atrophic rhinitis: a CFD study of air conditioning in the nasal cavity. J Appl Physiol. 2007;103(3):1082–92.
Masic I, Parojcic J, Djuric Z. Computational fluid dynamics: applications in pharmaceutical technology. InComputer-Aided Applications in Pharmaceutical Technology: Woodhead Publishing; 2013. p. 233–59.
Bai G, Bee JS, Biddlecombe JG, Chen Q, Leach WT. Computational fluid dynamics (CFD) insights into agitation stress methods in biopharmaceutical development. Int J Pharm [Internet]. 2012;423(2):264–80. Available from:. https://doi.org/10.1016/j.ijpharm.2011.11.044.
Tong ZB, Yang RY, Yu AB. CFD-DEM study of the aerosolisation mechanism of carrier-based formulations with high drug loadings. Powder Technol. 2017;314:620–6.
Benque B, Khinast JG. Understanding the motion of hard-shell capsules in dry powder inhalers. Int J Pharm [internet]. 2019;(June):118481. Available from:. https://doi.org/10.1016/j.ijpharm.2019.118481.
Coates MS, Fletcher DF, Chan HK, Raper JA. Effect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 1: grid structure and mouthpiece length. J Pharm Sci. 2004;93(11):2863–76.
Gallo-molina JP, Alvarez O. Multiscale analysis of w/o emulsions: a CFD approach. Ind Eng Chem Res. 2017:1–31.
Gallo-Molina JP, Ratkovich N, Alvarez O. The application of computational fluid dynamics to the multiscale study of oil-in-water emulsions. Ind Eng Chem Res. 2018;57(2):578–89.
Agterof WG, Vaessen GE, Haagh GA, Klahn JK, Janssen JJ. Prediction of emulsion particle sizes using a computational fluid dynamics approach. Colloids Surf B: Biointerfaces. 2003;31(1-4):141–8.
Pukkella AK, Vysyaraju R, Tammishetti V, Rai B, Subramanian S. Improved mixing of solid suspensions in stirred tanks with interface baffles: improved mixing of solid suspensions in stirred tanks with interface baffles: CFD simulation and experimental validation. Chem Eng J [internet]. 2018;15(358):621–33. Available from:. https://doi.org/10.1016/j.cej.2018.10.020.
Alvandimanesh AA, Sadrjavadi K. Optimization of de-esterified tragacanth microcapsules by computational fluid dynamic and the Taguchi design with purpose of the cell encapsulation. Int J Biol Macromol [internet]. 2017;1(105):17–26. Available from:. https://doi.org/10.1016/j.ijbiomac.2017.06.059.
Xing L, Li Y, Li T. Local Concentrating, Not Shear Stress, That May Lead to Possible Instability of Protein Molecules During Syringe Injection: A Fluid Dynamic Study with Two-Phase Flow Model. PDA J Pharm Sci Technol. 2019;73(3):260–75.
Zhu T, Moussa EM, Witting M, Zhou D, Sinha K, Hirth M, et al. Predictive models of lyophilization process for development, scale-up/tech transfer and manufacturing. Eur J Pharm Biopharm [Internet]. 2018;128(April):363–78. Available from:. https://doi.org/10.1016/j.ejpb.2018.05.005.
Zaborenko N, Shi Z, Corredor CC, Smith-goettler BM, Zhang L, Hermans A, et al. White paper first-principles and empirical approaches to predicting in vitro dissolution for pharmaceutical formulation and process development and for product release testing. AAPS J. 2019;21(3):1–20.
Tamrakar A, Devarampally DR, Ramachandran R. Advanced multiphase hybrid model development of fluidized bed wet granulation processes in Computer Aided Chemical Engineering, vol. 41: Elsevier; 2018. p. 159–87.
Guo Y, Wu CY. Computational modeling of pharmaceutical die filling processes. In: Predictive modeling of pharmaceutical unit operations: Cambridge: Woodhead Publishing; 2017. p. 253–71.
Turton R. The application of modeling techniques to film-coating processes film coating. Drug Dev Ind Pharm. 2010;36(2):143–51.
Kukura J, Arratia PE, Szalai ES, Muzzio FJ. Engineering tools for understanding the hydrodynamics of dissolution tests. Drug Dev Ind Pharm. 2003;29(2):231–9.
Kindgen S, Wachtel H, Abrahamsson B, Langguth P. Computational fluid dynamics simulation of hydrodynamics and stresses in the PhEur/USP disintegration tester under fed and fasted fluid characteristics. J Pharm Sci [internet]. 2015;104(9):2956–68. Available from:. https://doi.org/10.1002/jps.24511.
Fries L, Antonyuk S, Heinrich S, Dopfer D, Palzer S. Collision dynamics in fluidised bed granulators: a DEM-CFD study. Chem Eng Sci [internet]. 2013;86:108–23. Available from:. https://doi.org/10.1016/j.ces.2012.06.026.
Zarekar S, Bück A, Jacob M, Tsotsas E. Numerical study of the hydrodynamics of uidized beds operated under sub-atmospheric pressure. Chem Eng J [internet]. 2019;(372):1134–53. Available from:. https://doi.org/10.1016/j.cej.2019.04.159.
Toschkoff G, Suzzi D, Tritthart W, Reiter F, Schlingmann M, Khinast JG. Detailed analysis of air flow and spray loss in a pharmaceutical coating process. AICHE J. 2012;58(2):399–411.
Suzzi D, Radl S, Khinast JG. Local analysis of the tablet coating process: impact of operation conditions on film quality. Chem Eng Sci [internet]. 2010;65(21):5699–715. Available from:. https://doi.org/10.1016/j.ces.2010.07.007.
Kindgen S, Rach R, Nawroth T, Abrahamsson B, Langguth P. A novel disintegration tester for solid dosage forms enabling adjustable hydrodynamics. J Pharm Sci [internet]. 2016;105(8):2402–9. Available from:. https://doi.org/10.1016/j.xphs.2016.05.028.
Arcy DMD, Corrigan OI, Healy AM. Evaluation of hydrodynamics in the basket dissolution apparatus using computational fluid dynamics — dissolution rate implications. Eur J Pharm Sci. 2005;27:259–67.
Hapgood KP, Litster JD, White ET, Mort PR, Jones DG. Dimensionless spray flux in wet granulation: Monte-Carlo simulations and experimental validation. Powder Technol. 2004;141(1–2):20–30.
Iveson SM, Litster JD, Hapgood K, Ennis BJ. Nucleation, growth and breakage phenomena in agitated wet granulation processes: a review. Powder Technol. 2001;117(1–2):3–39.
Gjelstrup Kristensen H, Schaefer T. Granulation a review on pharmaceutical wet-granulation. Drug Dev Ind Pharm. 1987;13(4–5):803–72.
Tamrakar A, Devarampally DR, Ramachandran R. Advanced multiphase hybrid model development of fluidized bed wet granulation processes. In: Computer Aided Chemical Engineering. Elsevier; 2018;41:159–187.
Chua KW, Makkawi YT, Hounslow MJ. Time scale analysis for fluidized bed melt granulation I: granule-granule and granule-droplet collision rates. Chem Eng Sci [internet]. 2011;66(3):318–26. Available from:. https://doi.org/10.1016/j.ces.2010.10.033.
de Freitas LAP. Pharmaceutical applications of spouted beds: a review on solid dosage forms. Particuology [internet]. 2019;42:126–36. Available from:. https://doi.org/10.1016/j.partic.2018.05.002.
Duarte CR, Murata VV, Barrozo MAS. A study of the fluid dynamics of the spouted bed using CFD. Braz J Chem Eng. 2005;22(2):263–70.
Duarte CR, Murata VV, Barrozo MAS. Experimental and numerical study of spouted bed fluid dynamics. Braz J Chem Eng. 2008;25(1):95–107.
Vieira Neto JL, Duarte CR, Murata VV, Barrozo MAS. Effect of a draft tube on the fluid dynamics of a spouted bed: experimental and CFD studies. Dry Technol. 2008;26(3):299–307.
Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. 4th ed. Bombay: Varghese Publishing House; 2013. p. 89–107.
Thérèse S, Mortier FC, De Beer T, Gernaey KV, Paul J, Vervaet C, et al. Mechanistic modelling of fluidized bed drying processes of wet porous granules: a review. Eur J Pharm Biopharm. 2011;79:205–25.
Arastoopour H. Numerical simulation and experimental analysis of gas/solid flow systems: 1999 Fluor-Daniel plenary lecture. Powder Technol. 2001;119(2–3):59–67.
Jamaleddine TJ, Ray MB. Application of computational fluid dynamics for simulation of drying processes: a review. Dry Technol. 2010;28(2):120–54.
Baserinia R, Sinka IC. Powder die filling under gravity and suction fill mechanisms. Int J Pharm [internet]. 2019;563(January):135–55. Available from:. https://doi.org/10.1016/j.ijpharm.2019.01.068.
Mehta CH, Narayan R, Nayak UY. Computational modeling for formulation design. Drug Discov Today. 2019;24(3):781–8.
Guo Y, Kafui KD, Wu C-Y, CT. Pyrolysis of heavy oil in the presence of supercritical water: the reaction kinetics in different phases. Am Inst Chem Eng. 2015;55(1):49–62.
Wu CY, Guo Y. Numerical modelling of suction filling using DEM/CFD. Chem Eng Sci [internet]. 2012;73:231–8. Available from:. https://doi.org/10.1016/j.ces.2012.01.048.
Guo Y, Wu CY, Kafui KD, Thornton C. 3D DEM/CFD analysis of size-induced segregation during die filling. Powder Technol. 2011;206(1–2):177–88.
Toschkoff G, Khinast JG. Mathematical modeling of the coating process. Int J Pharm [internet]. 2013;457(2):407–22. Available from:. https://doi.org/10.1016/j.ijpharm.2013.08.022.
Pandey P, Song Y, Turton R. Chapter 8 Modelling of pan-coating processes for pharmaceutical dosage forms. Handb Powder Technol. 2007;11:377–416.
Karlsson S, Rasmuson A. CFD modeling of the Wurster bed coater. Am Inst Chem Eng. 2009;55(10):2578–90.
Liu M, Chen M, Li T, Tang Y, Liu R, Shao Y, et al. CFD–DEM–CVD multi-physical field coupling model for simulating particle coating process in spout bed. Particuology [internet]. 2019;42:67–78. Available from:. https://doi.org/10.1016/j.partic.2018.03.011.
Hilton JE, Ying DY, Cleary PW. Modelling spray coating using a combined CFD-DEM and spherical harmonic formulation. Chem Eng Sci [internet]. 2013;99:141–60. Available from:. https://doi.org/10.1016/j.ces.2013.05.051.
Quodbach J, Kleinebudde P. A critical review on tablet disintegration. Pharm Dev Technol [internet]. 2016;21(6):763–74. Available from:. https://doi.org/10.3109/10837450.2015.1045618.
Silva DA, Webster GK, Bou-Chacra N, Löbenberg R. The significance of disintegration testing in pharmaceutical development. Dissolution Technol. 2018;25(3):30–8.
Bai GE, Armenante PM, Plank RV, Gentzler M, Ford K, Harmon P. Hydrodynamic investigation of USP dissolution test apparatus II. J Pharm Sci. 2007;96(9):2327–49.
Todaro V, Persoons T, Grove G, Healy AM, D’Arcy DM. Characterization and simulation of hydrodynamics in the paddle, basket and flow-through dissolution testing apparatuses–a review. Dissolut Technol. 2017;24(3):24–36.
Bai G, Wang Y, Armenante PM. Velocity profiles and shear strain rate variability in the USP dissolution testing apparatus 2 at different impeller agitation speeds. Int J Pharm [internet]. 2011;403(1–2):1–14. Available from:. https://doi.org/10.1016/j.ijpharm.2010.09.022.
Mccarthy LG, Bradley G, Sexton JC, Corrigan OI, Healy AM. Computational fluid dynamics modeling of the paddle dissolution apparatus: agitation rate, mixing patterns, and fluid velocities. AAPS PharmSciTech. 2004;5(2):50–9.
Arcy DMD, Corrigan OI, Healy AM. Hydrodynamic simulation (computational fluid dynamics) of asymmetrically positioned tablets in the paddle dissolution apparatus: impact on dissolution rate and variability. J Pharm Pharmacol. 2005;57:1243–50.
Schiffter HA, Lee G. Single-droplet evaporation kinetics and particle formation in an acoustic levitator. Part 2: drying kinetics and particle formation from microdroplets of aqueous mannitol, trehalose, or catalase. J Pharm Sci. 2007;96(9):2284–95.
Arcy DMD, Liu B, Bradley G, Healy AM, Corrigan OI. Hydrodynamic and species transfer simulations in the USP 4 dissolution apparatus: considerations for dissolution in a low velocity pulsing flow. Pharm Res. 2010;27(2):246–58.
Wang B, Bredael G, Armenante PM. Computational hydrodynamic comparison of a mini vessel and a USP 2 dissolution testing system to predict the dynamic operating conditions for similarity of dissolution performance. Int J Pharm [internet]. 2018;539(1–2):112–30. Available from:. https://doi.org/10.1016/j.ijpharm.2018.01.002.
Kukura J, Arratia PC, Szalai ES, Bittorf KJ, Muzzio FJ. Understanding pharmaceutical flows. Pharm Technol. 2002;10(October):48–73.
Pragati K, Sharma HK. Concept of computational fluid dynamics (CFD) and its applications in food processing equipment design. J Food Process Technol. 2012;3(1):1–7.
Zhong W, Yu A, Zhou G, Xie J, Zhang H. CFD simulation of dense particulate reaction system: approaches, recent advances and applications. Chem Eng Sci [Internet]. 2016;140:16–43. Available from:. https://doi.org/10.1016/j.ces.2015.09.035.
Kremer DM, Hancock BC. Process simulation in the pharmaceutical industry: a review of some basic physical models. J Pharm Sci. 2006;95(3):517–29.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hemamanjushree, S., Tippavajhala, V.K. Simulation of Unit Operations in Formulation Development of Tablets Using Computational Fluid Dynamics. AAPS PharmSciTech 21, 103 (2020). https://doi.org/10.1208/s12249-020-1635-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-1635-1