Abstract
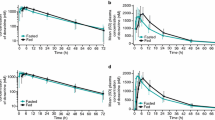
Doravirine is a non-nucleoside reverse transcriptase inhibitor indicated for the treatment of human immunodeficiency virus-1 infection, available as a single tablet in combination with other antiretroviral agents or as a fixed-dose regimen with lamivudine and tenofovir disoproxil fumarate (TDF). Alternative formulations of these drugs are being developed for individuals who have difficulty swallowing tablets. Two phase 1 trials were conducted, both in 24 healthy adults, to assess the pharmacokinetics of uncoated and coated oral granule formulations of doravirine, lamivudine, and TDF administered alone and with vanilla pudding or apple sauce. The pharmacokinetics for all uncoated granules, and of coated lamivudine and TDF granules, were similar to those of currently marketed tablets (geometric mean ratios [GMRs] 0.92–1.04). Coated doravirine granules had decreased AUC0–∞ (11%) and Cmax (23%) values versus the tablet. The pharmacokinetics were similar for uncoated and coated doravirine granules administered with or without pudding (GMRs 0.96–1.10); administration with apple sauce increased doravirine AUC0–∞ (26–29%), Cmax (56–59%), and C24 (20–21%) versus administration of granules alone. Lamivudine granules administered with pudding or apple sauce decreased AUC0–∞ and Cmax (14–25%) versus granules alone. Tenofovir AUC0–∞, Cmax, and C24 increased for TDF granules administered with pudding or apple sauce versus alone (11–23%). Pharmacokinetic differences when administering doravirine, lamivudine, or TDF as uncoated or coated granules versus tablets, or when granules were administered with (versus without) pudding or apple sauce, are not considered clinically meaningful, supporting further development of these granule formulations.

Similar content being viewed by others
References
UNAIDS. Global HIV & AIDS statistics - 2019 fact sheet. 2019. Available online: http://www.unaids.org/en/resources/fact-sheet. Accessed 19 August 2019.
UNAIDS. UNAIDS Data 2018. 2018. Available online: http://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf. Accessed 19 August 2019.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. 2018. Available online: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 19 August 2019.
Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. 2018. Available online: https://aidsinfo.nih.gov/contentfiles/lvguidelines/PediatricGuidelines.pdf. Accessed 19 August 2019.
Merck Sharp & Dohme Corp. DELSTRIGO™ (doravirine, lamivudine, and tenofovir disoproxil fumarate) Prescribing Information. Merck & Co., Inc., Whitehouse Station, NJ, USA. 2019. Available online: https://www.merck.com/product/usa/pi_circulars/d/delstrigo/delstrigo_pi.pdf. Accessed 4 October 2019.
Merck Sharp & Dohme Corp. PIFELTRO™ (doravirine) Prescribing Information. Merck & Co., Inc., Whitehouse Station, NJ, USA. 2019. Available online: https://www.merck.com/product/usa/pi_circulars/p/pifeltro/pifeltro_pi.pdf. Accessed 4 October 2019.
Molina JM, Squires K, Sax PE, Cahn P, Lombaard J, DeJesus E, et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV. 2018;5(5):e211–e20.
Orkin C, Squires KE, Molina JM, Sax PE, Wong WW, Sussmann O, et al. Doravirine/lamivudine/tenofovir disoproxil fumarate is non-inferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive adults with human immunodeficiency virus-1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis. 2019;68(4):535–44.
Molina J-M, Squires KE, Sax P, Cahn P, Lombaard J, DeJesus E, et al. Doravirine (DOR) versus ritonavir-boosted darunavir (DRV+r): 96-week results of the randomized, double-blind, phase 3 DRIVE-FORWARD Noninferiority trial. Abstract LBPEB017, 22nd International AIDS Conference, 2018. Available online: http://www.aids2018.org/Portals/4/File/AIDS2018_Abstract_book.pdf. Accessed 19 August 2019.
Gatell JM, Morales-Ramirez JO, Hagins DP, Thompson M, Arastéh K, Hoffmann C, et al. Doravirine dose selection and 96-week safety and efficacy versus efavirenz in antiretroviral therapy-naive adults with HIV-1 infection in a Phase IIb trial. Antiviral Ther. 2019;24(6):425–35.
Sanchez RI, Fillgrove KL, Yee KL, Liang Y, Lu B, Tatavarti A, et al. Characterisation of the absorption, distribution, metabolism, excretion and mass balance of doravirine, a non-nucleoside reverse transcriptase inhibitor in humans. Xenobiotica. 2019;49(4):422–32.
Khalilieh S, Yee KL, Sanchez RI, Triantafyllou I, Fan L, Maklad N, et al. Results of a doravirine-atorvastatin drug-drug interaction study. Antimicrob Agents Chemother. 2017;61(2):e01364–16.
Anderson MS, Khalilieh S, Yee KL, Liu R, Fan L, Rizk ML, et al. A two-way steady-state pharmacokinetic interaction study of doravirine (MK-1439) and dolutegravir. Clin Pharmacokinetics. 2017;56(6):661–9.
Bleasby K, Fillgrove KL, Houle R, Lu B, Palamanda J, Newton DJ, et al. In vitro evaluation of the drug interaction potential of doravirine. Antimicrob Agents Chemother. 2019;63(4):e02492–18.
Anderson MS, Gilmartin J, Fan L, Yee KL, Kraft WK, Triantafyllou I, et al. No meaningful drug interactions with doravirine, lamivudine and tenofovir disoproxil fumarate co-administration. Antivir Ther. 2019;24(6):443–50.
Behm MO, Yee KL, Liu R, Levine V, Panebianco D, Fackler P. The effect of food on doravirine bioavailability: results from two pharmacokinetic studies in healthy subjects. Clin Drug Investig. 2017;37(6):571–9.
GlaxoSmithKline/ViiV Healthcare. EPIVIR (lamivudine) Prescribing Information. 2019. Available online: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Epivir/pdf/EPIVIR-PI-PIL.PDF. Accessed 19 August 2019.
Gilead Sciences, Inc. VIREAD® (tenofovir disproxil fumarate) Prescribing Information. 2018. Available online: http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/viread/viread_pi.pdf. Accessed 19 August 2019.
Lopez FL, Ernest TB, Tuleu C, Gul MO. Formulation approaches to pediatric oral drug delivery: benefits and limitations of current platforms. Expert Opin Drug Deliv. 2015;12(11):1727–40.
Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983–97.
Food and Drug Administration (FDA). Use of liquids and/or soft foods as vehicles for drug administration: general considerations for selection and in vitro methods for product quality assessments - guidance for industry. 2018. Available online: https://www.fda.gov/media/114872/download. Accessed 26 November 2019.
Ankrom W, Sanchez RI, Yee KL, Fan L, Mitra P, Wolford D, et al. Investigation of pharmacokinetic interactions between doravirine and elbasvir/grazoprevir and ledipasvir/sofosbuvir. Antimicrob Agents Chemother. 2019;63:e02491–18(5).
Anderson MS, Gilmartin J, Cilissen C, De Lepeleire I, van Bortel L, Dockendorf MF, et al. Safety, tolerability and pharmacokinetics of doravirine, a novel HIV non-nucleoside reverse transcriptase inhibitor, after single and multiple doses in healthy subjects. Antivir Ther. 2015;20(4):397–405.
Khalilieh SG, Yee KL, Fan L, Liu R, Heber W, Dunzo E, et al. A randomized trial to assess the effect of doravirine on the QTc interval using a single supratherapeutic dose in healthy adult volunteers. Clin Drug Investig. 2017;37(10):975–84.
Angel JB, Hussey EK, Hall ST, Donn KH, Morris DM, McCormack JP, et al. Pharmacokinetics of 3TC (GR109714X) administered with and without food to HIV-infected patients. Drug Invest. 1993;6(2):70–4.
Kasirye P, Kendall L, Adkison KK, Tumusiime C, Ssenyonga M, Bakeera-Kitaka S, et al. Pharmacokinetics of antiretroviral drug varies with formulation in the target population of children with HIV-1. Clin Pharmacol Ther. 2012;91(2):272–80.
Acknowledgments
The authors would like to thank the trial participants and staff. In addition, the authors would like to thank Marty Behm for his help with the study, Robert Valesky for bioanalytical support, and Karen Thompson for input regarding formulation development.
Medical writing assistance, under the direction of the authors, was provided by Kirsty Muirhead, PhD, of CMC AFFINITY, McCann Health Medical Communications, and Claire Lavin, PhD, on behalf of CMC AFFINITY, in accordance with Good Publication Practice (GPP3) guidelines. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Funding
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KLY, ADB, LF, SK, IT, M-HV, PF, SAS, and MI are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 37 kb)
Rights and permissions
About this article
Cite this article
Yee, K.L., DiBenedetto, A., Fan, L. et al. Comparative Bioavailability of Oral Granule Formulations of the HIV Antiretroviral Drugs Doravirine, Lamivudine, and Tenofovir Disoproxil Fumarate. AAPS PharmSciTech 21, 91 (2020). https://doi.org/10.1208/s12249-020-1630-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-1630-6