Abstract
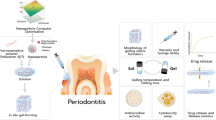
The objectives of the present research work were systematic development of novel in situ gel formulation containing nanoparticles for localised delivery of moxifloxacin against bacterial periodontitis. PLGA nanoparticles were prepared and optimised in a systematic manner. Factor screening was performed with the help of half-factorial design to identify the influential factors, while response surface optimisation of the nanoparticles was conducted using central composite design. The optimum nanoparticle formulation was chosen on the basis of lower particle size, higher drug entrapment and controlled drug release characteristics up to 1 week time period, while the optimum in situ gel was selected on the basis of faster gelling and higher viscosity and gel strength properties for improved retention in the periodontium. In vivo histopathological studies and in vivo gamma scintigraphy studies revealed the extended release, superior efficacy and enhanced retention of nanoparticle-loaded in situ gelling system. Results obtained from in vivo histopathological studies after 1 week treatment with in situ gel formulation containing nanoparticles of moxifloxacin were found to be better than with 3 weeks treatment of marketed gel formulation. Overall, the studies ratify successful development of an effective site-specific drug delivery system with enhanced biopharmaceutical attributes for the periodontitis treatment.









Similar content being viewed by others
References
AlJehani YA. Risk factors of periodontal disease: review of the literature. Int J Dent. 2014;2014:182513.
Geskovski N, Sazdovska SD, Gjosheva S, Petkovska R, Popovska M, Anastasova L, et al. Rational development of nanomedicines for molecular targeting in periodontal disease. Arch Oral Biol. 2018;93:31–46.
Jain N, Jain GK, Javed S, Iqbal Z, Talegaonkar S, Ahmad FJ, et al. Recent approaches for the treatment of periodontitis. Drug Discov Today. 2008;13(21–22):932–43.
Joshi D, Garg T, Goyal AK, Rath G. Advanced drug delivery approaches against periodontitis. Drug Deliv. 2014;23(2):363–77.
Pinon-Segundo E, Ganem-Quintanar A, Alonso-Perez V, Quintanar-Guerrero D. Preparation and characterization of triclosan nanoparticles for periodontal treatment. Int J Pharm. 2005;294(1–2):217–32.
Dabhi MR, Nagori SA, Gohel MC, Parikh RK, Sheth NR. Formulation development of smart gel periodontal drug delivery system for local delivery of chemotherapeutic agents with application of experimental design. Drug Deliv. 2010;17(7):520–31.
Mudgil M, Pawar PK. Preparation and in vitro/ex vivo evaluation of Moxifloxacin-loaded PLGA Nanosuspensions for ophthalmic application. Sci Pharm. 2013;81(2):591–606.
Guentsch A, Jentsch H, Pfister W, Hoffmann T, Eick S. Moxifloxacin as an adjunctive antibiotic in the treatment of severe chronic periodontitis. J Periodontol. 2008;79(10):1894–903.
Ardila CM, Lopez MA, Guzman IC. High resistance against clindamycin, metronidazole and amoxicillin in Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans isolates of periodontal disease. Med Oral Patol Oral Cir Bucal. 2010;15(6):947–51.
Caeiro JP, Iannini PB. Moxifloxacin (Avelox): a novel fluoroquinolone with a broad spectrum of activity. Expert Rev Anti-Infect Ther. 2003;1(3):363–70.
Flemmig TF, Petersilka G, Volp A, Gravemeier M, Zilly M, Mross D, et al. Efficacy and safety of adjunctive local moxifloxacin delivery in the treatment of periodontitis. J Periodontol. 2010;82(1):96–105.
Swain GP, Patel S, Gandhi J, Shah P. Development of moxifloxacin hydrochloride loaded in-situ gel for the treatment of periodontitis: in-vitro drug release study and antibacterial activity. J Oral Biol Craniofac Res. 2019;9(3):190–200.
Manjunath S, Ravindra S, Ahmed M, Naveen R, Oswal S, Rangaraju V. Moxifloxacin in situ gel as an adjunct in the treatment of periodontal pocket: a clinico-microbiological study. Int J Oral Health Sci. 2018;8(1):19–25.
Shah S, Upadhyay P, Parikh D, Shah J. In situ gel: a novel approach of gastroretentive drug delivery. Asian J Biomed Pharm Sci. 2012;2(8):1–8.
Prajapati VD, Jani GK, Khutliwala TA, Zala BS. Raft forming system-an upcoming approach of gastroretentive drug delivery system. J Control Release. 2013;168(2):151–65.
Kouchak M. In situ gelling systems for drug delivery. Jundishapur J Nat Pharm Prod. 2014;9(3):e20126-e.
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B: Biointerfaces. 2010;75(1):1–18.
Beg S, Rahman M, Barkat M, Ahmad FJ. Nanomedicine for the treatment of disease: from concept to application. New York: Apple Academic Press; 2019.
Kranti K, Mani R, Tervankar AR. Effectiveness of subgingivally delivered 0.4% moxifloxacin gel and 0.1% oflaxacin gel in the treatment of chronic periodontitis. Int J Dent Oral Heal. 2016;2(1):8–13.
Sheshala R, Quah SY, Tan GC, Meka VS, Jnanendrappa N, Sahu PS. Investigation on solution-to-gel characteristic of thermosensitive and mucoadhesive biopolymers for the development of moxifloxacin-loaded sustained release periodontal in situ gels. Drug Deliv Transl Res. 2019;9(2):434–43.
Beg S, Hasnain MS, Rahman M, Swain S. Chapter 1. Introduction to quality by design (QbD): fundamentals, principles, and applications. In: Beg S, Hasnain MS, editors. Pharmaceutical quality by design: Academic Press; 2019. p. 1–17.
Beg S, Swain S, Rahman M, Hasnain MS, Imam SS. Chapter 3. Application of design of experiments (DoE) in pharmaceutical product and process optimization. In: Beg S, Hasnain MS, editors. Pharmaceutical quality by design: Academic Press; 2019. p. 43–64.
Nayak AK, Ahmed SA, Beg S, Tabish M, Hasnain MS. Chapter 18. Application of quality by design for the development of biopharmaceuticals. In: Beg S, Hasnain MS, editors. Pharmaceutical quality by design: Academic Press; 2019. p. 399–411.
Beg S, Rahman M, Panda SS. Pharmaceutical QbD: omnipresence in the product development lifecycle. Eur Pharm Rev. 2017;22(1):58–64.
Singh B, Beg S. Quality by design in product development life cycle. Chron Pharmabiz. 2013;22:72–9.
Hasnain MS, Ahmed SA, Beg S, Ansari MT, Nayak AK. Chapter 15. Quality by design approach for development of multiparticulate drug delivery systems. In: Beg S, Hasnain MS, editors. Pharmaceutical quality by design: Academic Press; 2019. p. 351–65.
Swain S, Jena BR, Madugula D, Beg S, Hasnain MS. Chapter 6. Application of quality by design paradigms for development of solid dosage forms. In: Beg S, Hasnain MS, editors. Pharmaceutical quality by design: Academic Press; 2019. p. 109–30.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16(4):771–83.
Lionberger RA, Lee SL, Lee L, Raw A, Yu LX. Quality by design: concepts for ANDAs. AAPS J. 2008;10(2):268–76.
Singh B, Kapil R, Nandi M, Ahuja N. Developing oral drug delivery systems using formulation by design: vital precepts, retrospect and prospects. Expert Opin Drug Deliv. 2011;8(10):1341–60.
Beg S, Hasnain MS. Pharmaceutical quality by design: principles and applications. New York: Academic Press (Elsevier); 2019.
Singh B, Beg S, Raza K. Developing “optimized” drug products employing “designed” experiments. Chem Ind Digest. 2013;23:70–6.
Negi P, Singh B, Sharma G, Beg S, Katare OP. Biocompatible lidocaine and prilocaine loaded-nanoemulsion system for enhanced percutaneous absorption: QbD-based optimisation, dermatokinetics and in vivo evaluation. J Drug Target. 2015;32(5):419–31.
Beg S, Rahman M, Kohli K. Quality-by-design approach as a systematic tool for the development of nanopharmaceutical products. Drug Discov Today. 2019;24(3):717–25.
Beg S, Saini S, Bandopadhyay S, Katare OP, Singh B. QbD-driven development and evaluation of nanostructured lipid carriers (NLCs) of Olmesartan medoxomil employing multivariate statistical techniques. Drug Dev Ind Pharm. 2017;44(3):407–20.
Bansal S, Beg S, Garg B, Asthana A, Asthana GS, Singh B. QbD-oriented development and characterization of effervescent floating-bioadhesive tablets of cefuroxime axetil. AAPS PharmSciTech. 2015;17(5):1086–99.
Bansal S, Beg S, Asthana A, Garg B, Asthana GS, Kapil R, et al. QbD-enabled systematic development of gastroretentive multiple-unit microballoons of itopride hydrochloride. Drug Deliv. 2014;23(2):437–51.
Negi P, Singh B, Sharma G, Beg S, Raza K, Katare OP. Phospholipid microemulsion-based hydrogel for enhanced topical delivery of lidocaine and prilocaine: QbD-based development and evaluation. Drug Deliv. 2014;23(3):951–67.
Beg S, Sharma G, Thanki K, Jain S, Katare OP, Singh B. Positively charged self-nanoemulsifying oily formulations of olmesartan medoxomil: systematic development, in vitro, ex vivo and in vivo evaluation. Int J Pharm. 2015;493(1–2):466–82.
Garg V, Singh H, Bhatia A, Raza K, Singh SK, Singh B, et al. Systematic development of transethosomal gel system of piroxicam: formulation optimization, in vitro evaluation, and ex vivo assessment. AAPS PharmSciTech. 2016;18(1):58–71.
Beg S, Raza K, Kumar R, Chadha R, Katare OP, Singh B. Improved intestinal lymphatic drug targeting via phospholipid complex-loaded nanolipospheres of rosuvastatin calcium. RSC Adv. 2016;6(10):8173–87.
Rao JP, Geckeler KE. Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci. 2011;36(7):887–913.
Song X, Zhao Y, Hou S, Xu F, Zhao R, He J, et al. Dual agents loaded PLGA nanoparticles: systematic study of particle size and drug entrapment efficiency. Eur J Pharm Biopharm. 2008;69(2):445–53.
Singh B, Raza K, Singhal P, Wadhwa S, Katare OP. Systematically optimized biocompatible isotretinoin-loaded solid lipid nanoparticles (SLNs) for topical treatment of acne. Colloids Surf B: Biointerfaces. 2013;105:67–74.
Koland M, Charyulu RN, Vijayanarayana K, Prabhu P. In vitro and in vivo evaluation of chitosan buccal films of ondansetron hydrochloride. Int J Pharm Investig. 2011;1(3):164–71.
Singh B, Kaur T, Singh S. Correction of raw dissolution data for loss of drug during sampling. Indian J Pharm Sci. 1997;59:196–9.
Singh B, Singh S. A comprehensive computer program for study of drug release kinetics from compressed matrices. Indian J Pharm Sci. 1998;60:313–6.
Pandey P, Rahman M, Bhatt PC, Beg S, Paul B, Hafeez A, et al. Implication of nano-antioxidant therapy for treatment of hepatocellular carcinoma using PLGA nanoparticles of rutin. Nanomedicine. 2018;13(8):849–70.
Li Z, Ning W, Wang J, Choi A, Lee P-Y, Tyagi P, et al. Controlled gene delivery system based on thermosensitive biodegradable hydrogel. Pharm Res. 2003;20(6):884–8.
Botelho MA, Martins JG, Ruela RS, Queiroz DB, Ruela WS. Nanotechnology in ligature-induced periodontitis: protective effect of a doxycycline gel with nanoparticules. J Appl Oral Sci. 2010;18(4):335–42.
Leite CLA, Redins CA, Vasquez EC, Meyrelles SS. Experimental-induced periodontitis is exacerbated in spontaneously hypertensive rats. Clin Exp Hypertens. 2005;27(6):523–31.
Lima V, Vidal FDP, Rocha FAC, Brito GAC, Ribeiro RA. Effects of tumor necrosis factor—inhibitors pentoxifylline and thalidomide on alveolar bone loss in short-term experimental periodontal disease in rats. J Periodontol. 2004;75(1):162–8.
Carvalho RS, de Souza CM, Neves JCS, Holanda-Pinto SA, Pinto LMS, Brito GAC, et al. Research effect of venlafaxine on bone loss associated with ligature-induced periodontitis in Wistar rats. J Negat Results Biomed. 2010;9(3):2–8.
Schwartz J, Connor R. Optimization techniques in pharmaceutical formulation and processing. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. New York: Marcel Dekker, Inc.; 1996. p. 943.
Singh B, Dahiya M, Saharan V, Ahuja N. Optimizing drug delivery systems using systematic design of experiments. Part II: retrospect and prospects. Crit Rev Ther Drug Carrier Syst. 2005b;22(3):215–93.
Swider E, Koshkina O, Tel J, Cruz LJ, de Vries IJM, Srinivas M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018;73:38–51.
Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011;3(3):1377–97.
Yang Q, Owusu-Ababio G. Biodegradable progesterone microsphere delivery system for osteoporosis therapy. Drug Dev Ind Pharm. 2000;26(1):61–70.
Rahman Z, Zidan AS, Habib MJ, Khan MA. Understanding the quality of protein loaded PLGA nanoparticles variability by Plackett-Burman design. Int J Pharm. 2010;389(1–2):186–94.
Fattal E, Roques B, Puisieux F, Blanco PMJ, Couvreur P. Multiple emulsion technology for the design of microspheres containing peptides and oligopeptides. Adv Drug Deliv Rev. 1997;28(1):85–96.
Singh B, Kumar R, Ahuja N. Optimizing drug delivery systems using systematic "design of experiments." part I: fundamental aspects. Crit Rev Ther Drug Carrier Syst. 2005;22(1):27–105.
Lutrol F127—technical information. Available online: http://www.rumapel.com.ar/pharma_excipientes/ficha_tecnica/Lutrol%20F127.pdf. Accessed 12 December 2019.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23.
Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev. 2008;37(8):1473–81.
Wahl SM, Costa GL, Mizel DE, Allen JB, Skaleric U, Mangan DF. Role of transforming growth factor beta in the pathophysiology of chronic inflammation. J Periodontol. 1993;64(5 Suppl):450–5.
Williams RC. Periodontal disease. N Engl J Med. 1990;322(6):373–82.
Acknowledgements
The authors also wish to acknowledge the complementary perpetual license granted by Stat-Ease Inc., USA to the author Bhpinder Singh as a part of “Pharma QbD Award” for his unparallel contribution towards QbD-based development of optimized drug delivery systems and pharmaceutical processes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 206 kb)
Rights and permissions
About this article
Cite this article
Beg, S., Dhiman, S., Sharma, T. et al. Stimuli Responsive In Situ Gelling Systems Loaded with PLGA Nanoparticles of Moxifloxacin Hydrochloride for Effective Treatment of Periodontitis. AAPS PharmSciTech 21, 76 (2020). https://doi.org/10.1208/s12249-019-1613-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1613-7