Abstract
Hypertension shows circadian blood pressure rhythms (day–night pattern) that urge the delivery of antihypertensive drugs at the right time in the desired levels. Thus, a bilayered core-in-cup buccoadhesive tablet was formulated that immediately releases olmesartan, to give a burst effect, and controls azelnidipine release, to prolong its therapeutic effect. The main challenge was the poor bioavailability of azelnidipine due to its poor aqueous solubility and first-pass effect. Hence, liquisolid compact buccoadhesive tablets were prepared to enhance solubility, dissolution profiles, and bypass the oral route. Two factorial designs were conducted to study the type and concentration effect of the mucoadhesive polymers on the dissolution and mucoadhesion of olmesartan and azelnidipine. Characterization studies were conducted regarding drug content, surface pH, water uptake, mucoadhesive strength, in vitro release, and ex vivo permeability. The core-in-cup olmesartan/azelnidipine buccoadhesive tablet showed similar release profile to the statistically optimized formulae of each drug. In vitro dissolution study showed enhanced release of azelnidipine than the directly compressed tablets, to comply with the regulatory standards of controlled release systems. In vivo pharmacokinetic study of olmesartan and azelnidipine conducted on human volunteers against Rezaltas® 10/8 mg tablet showed percentage relative bioavailability of 106.12 and 470.82%, respectively.

Graphical Abstract





Similar content being viewed by others
References
Modi D, Amaliyar P, Kalal Y, Gangadia B, Chaudhry S, Sanghvi K, et al. Novel approach in compressed-coated tablet dosage form: core-in-cup (in lay) tablet with geometrically altered drug delivery concept. Bri Bio Bull. 2013;1(1):90–102.
Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2006;19(11):1190–6.
Kjeldsen SE, Aksnes TA, de la Sierra A, Ruilope LM. Amlodipine and valsartan: calcium channel blockers/angiotensin II receptor blockers combination for hypertension. Therapy. 2007;4(1):31–40.
Prabhakar D, Sreekanth J, Jayaveera K. Development and evaluation of transdermal patches of azelnidipine. Int J Pharm Pharm Sci. 2013;5:805.
Wellington K, Scott LJ. Azelnidipine. Drugs. 2003;63(23):2613–21.
Han Y, Pan Y, Lv J, Guo W, Wang J. Powder grinding preparation of co-amorphous β-azelnidipine and maleic acid combination: molecular interactions and physicochemical properties. Powder Technol. 2016;291:110–20.
Hamsanandini J, Parthiban S, Vikneswari A, Tamiz MT. Dissolution enhancement techniques of poorly soluble drugs by liquisolid compacts. Int J Res Pharm Nano Sci. 2014;3(4):298–304.
Shivanand K, Raju SA, Jaykar B. Mucoadhessive bilayered buccal tablets of tizanidine hydrochloride. Int J Pharm Tech Res. 2010;2(3):1861–9.
Jadhav TS, Thombre NA, Kshirsagar SJ. Pulsatile drug delivery system using core-in-cup approach: a review. Pharm Biol Eval. 2016;3(3):288–304.
British Pharmacopeia. 2015.
Basalious EB, El-Sebaie W, El-Gazayerly O. Application of pharmaceutical QbD for enhancement of the solubility and dissolution of a class II BCS drug using polymeric surfactants and crystallization inhibitors: development of controlled-release tablets. AAPS PharmSci Tech. 2011:9646–66.
Patel JK, Patel NK. Validated stability-indicating RP-HPLC method for the simultaneous determinat ion of azelnidipine and olmesartan in their combined dosage form. Sci Pharm. 2014;82(1):541–54.
Singh R, Sharma D, Garg R. Review on mucoadhesive drug delivery system with special emphasis on buccal route: an important tool in designing of novel controlled drug delivery system for the effective delivery of pharmaceuticals. J Dev Drugs. 2017;6(1):1–12.
Tangri P, Jawla S, Jakhmola V, Mishra R. Highlights of mucoadhesive drug delivery systems: a review. Indo Glob J Pharm. 2017;7(2):112–25.
European Medicines Agency. Guideline on quality of oral modified release products. 2012.
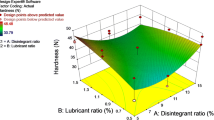
Shah M, Pathak K. Development and statistical optimization of solid lipid nanoparticles of simvastatin by using 23 full-factorial design. AAPS PharmSciTech. 2010;11(2):489–96.
Singh B, Mehta G, Kumar R, Bhatia A, Ahuja N, Katare O. Design, development and optimization of nimesulide-loaded liposomal systems for topical application. Curr Drug Deliv. 2005;2(2):143–53.
Swarupa A, Sharma JVC, Vishnu Priya P, Shyamala. A review on liquisolid technology. Int J Pharm Sci Rev Res. 2015;5(3):207–11.
Draganoiu E, Rajabi-Siahboomi AR, Tiwari SB. Handbook of pharmaceutical excipients. 6th ed. London: Pharmaceutical Press; 2009.
Darekar SS, Khadabadi SS, Shahi SR. Formulation and evaluation of bilayer buccal tablet of sumatriptan succinate. Int J Pharm Pharm Sci. 2014;6(6):469–75.
Patil GB, Patil ND, Deshmukh PK, Patil PO, Bari SB. Nanostructured lipid carriers as a potential vehicle for carvedilol delivery: application of factorial design approach. Artificial Cells, Nanomedicine, and Biotechnology. 2016;44(1):12–9.
Parodi B, Russo E, Caviglioli G, Cafaggi S, Bignardi G. Development and characterization of a buccoadhesive dosage form of oxycodone hydrochloride. Drug Dev Ind Pharm. 1996;22(5):445–50.
Çelik B. Risperidone mucoadhesive buccal tablets: formulation design, optimization and evaluation. Drug Des Devel Ther. 2017;11:3355–65.
Olorunsola EO, Akpan GA, Adikwu MU. Evaluation of chitosan-microcrystalline cellulose blends as direct compression excipients. J Drug Deliv. 2017;2017:1–8.
Kassem MAA, ElMeshad AN, Fares AR. Enhanced bioavailability of buspirone hydrochloride via cup and corebuccal tablets: formulation and in vitro/in vivo evaluation. Int J Pharm. 2014;463(1):68–80.
Langoth N, Kalbe J, Bernkop-Schnürch A. Development of buccal drug delivery systems based on a thiolated polymer. Int J Pharm. 2003;252(1–2):141–8.
Jenkins S, Liversidge GG. Nanoparticulate azelnidipine formulations. 2010; US20100221327 A1. p.1–24.
Moore JW. Mathematical comparison of dissolution profiles. Pharm Technol. 1996;20:64–75.
FDA. Guidance for industry: dissolution testing of immediate release solid oral dosage forms. 1997.
Higuchi T. Mechanisms of sustained action medication,theoretical analysis of the rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52(1):1145–9.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm. 1983;72(10):1189–91.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23.
El-Samaligy M, Yahia S, Basalious E. Formulation and evaluation of diclofenac sodium buccoadhesive discs. Int J Pharm. 2004;286(1–2):27–39.
FDA. Guidance for industry, bioavailability and bioequivalence studies submitted in NDAs or INDs—general considerations 2014.
FDA. Guidance of industry, food-effect bioavailability and bioequivalence studies. 2002.
Ramesh A, Neelima B, Pallapothu L, Ashok Kumar A. A novel method for the simultaneous determination of azelnidipine and olmesartan in human plasma by using liquid chromatography-electro spray ionization tandem mass spectrometry and application to a pharmacokinetic study. Int J Pharm. 2017;7(3):111–24.
Perioli L, Ambrogi V, Giovagnoli S, Blasi P, Mancini A, Ricci M, et al. Influence of compression force on the behavior of mucoadhesive buccal tablets. AAPS Pharm SciTech. 2008;9(1):274–81.
Elshafeey AH, Kamel AO, Awad GAS. Ammonium methacrylate units polymer content and their effect on acyclovir colloidal nanoparticles properties and bioavailability in human volunteers. Colloids Surf B. 2010;75(2):398–404.
Maryadele, O’Neil J. The Merck index. 14th ed. Whitehouse Station: Merck & Co., Inc.; 2006.
Sruthy PN, Anoop KR. Formulation and evaluation of olmesartan medoxomil floating tablets. Int J Pharm Pharm Sci. 2013;5(3):691–6.
Kapavarapu S, Bhandaru VR, Chintala R. Structural identification and characterization of potential impurities of azelnidipine. Int J Pharm Res Scholars. 2016;5(1–2):202–17.
Damian F, Blaton N, Naesens L, Balzarini J, Kinget R, Augustijns P, et al. Physicochemical characterization of solid dispersions of the antiviral agent UC-781 with polyethylene glycol 6000 and Gelucire 44/14. Eur J Pharm Sci. 2000;10(4):311–22.
Aditya G, Gudas GK, Bingi M, Debnath S, Rajesham VV. Design and evaluation of controlled release mucoadhesive buccal tablets of lisinopril. Int J Curr Pharm Res. 2010;2(4):24–7.
Patel VM, Prajapati BG, Patel MM. Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranololhydrochloride. AAPS PharmSciTech. 2007;8(1):147–54.
Ali MF, Bakalisa S. Mucoadhesive polymers for food formulations. Procedia Food Sci. 2011;1(1):68–75.
Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
Srivalli KMR, Lakshmi PK, Balasubramaniam J. Design of a novel bilayered gastric mucoadhesive system for localized and unidirectional release of lamotrigine. Saudi Pharm J. 2013;21(1):45–52.
Raju KN, Velmurugan S, Deepika B, Vinushitha S. Formulation and in vitro evaluation of buccal tablets of metoprolol tartrate. Int J Pharm Pharm Sci. 2011;3(2):239–46.
Habib BS, Abd El Rehim RT, Nour SA. Feasibility of optimizing trimetazidine dihydrochloride release from controlled porosity osmotic pump tablets of directly compressed cores. J Adv Res. 2014;5(1):347–56.
Chen J, Qiu L, Hu M, Jin Y, Han J. Preparation, characterization and in vitro evaluation of solid dispersions containing docetaxel. Drug Dev Ind Pharm. 2008;34(6):588–94.
Delaney SP, Nethercott MJ, Mays CJ, Winquist NT, Arthur D, Calahan JL, et al. Characterization of synthesized and commercial forms of magnesium stearate using differential scanning calorimetry, thermogravimetric analysis, powder X-ray diffraction, and solid-state NMR spectroscopy. J Pharm Sci. 2017;106(1):338–47.
Xie Y, Li G, Yuan X, Cai Z, Rong R. Preparation and in vitro evaluation of solid dispersions of total flavones of Hippophae rhamnoides L. AAPS PharmSciTech. 2009;10(2):631–40.
Rojas J, Lopez A, Guisao S, Ortiz C. Evaluation of several microcrystalline celluloses obtained from agricultural by-products. Journal of Advanced Pharmaceutical Technology & Research. 2011;2(3):144–50.
Roberts SNC, Williams AC, Grimsey IM, Booth SW. Quantitative analysis of mannitol polymorphs. X-ray powder diffractometry—exploring preferred orientation effects. J Pharm Biomed Anal. 2002;28(6):1149–59.
Mogilevskaya E, Akopova T, Zelenetskii A, Ozerin A. The crystal structure of chitin and chitosan. Polymer Science Series A. 2006;48(2):116–23.
Dondeti P, Zia H, Needham TE. Bioadhesive and formulation parameters affecting nasal absorption. Int J Pharm. 1996;127(2):115–33.
Wang J, Sakai S, Deguchi Y, Bi D, Tabata Y, Morimoto K. Aminated gelatin as a nasal absorption enhancer for peptide drugs: evaluation of absorption enhancing effect and nasal mucosa perturbation in rats. J Pharm Pharmacol. 2002;54(2):181–8.
Mohanty D, Gurulatha C, Bakshi V, Mavya B. Novel aproaches on buccal mucoadhesive drug delivery system. Indo Am J Pharm Res. 2018;5(4):2131–45.
Illum L, Jørgensen H, Bisgaard H, Krogsgaard O, Rossing N. Bioadhesive microspheres as a potential nasal drug delivery system. Int J Pharm. 1987;39(3):189–99.
Nakamura K, Maitani Y, Lowman AM, Takayama K, Peppas NA, Nagai T. Uptake and release of budesonide from mucoadhesive, pH-sensitive copolymers and their application to nasal delivery. Journal of Controlled Release: official journal of the Controlled Release Society. 1999;61(3):329–35.
Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: the next generation. J Pharm Sci. 2000;89(7):850–66.
Esim O, Savaser A, Ozkan CK, Bayrak Z, Tas C, Ozkan Y. Effect of polymer type on characteristics of buccal tablets using factorial design. Saudi Pharm J. 2018;26(1):53–63.
Chatterjee B, Amalina N, Sengupta P, Mandal UK. Mucoadhesive polymers and their mode of action: a recent update. J Appl Pharm Sci. 2017;7(5):195–203.
Singh MK, Prajapati SK, Mahor A, Rajput N, Singh R. Formulation and in-vitro evaluation of microcrystalline chitosan based buccoadhesive bilayered tablets of repaglinide. Int J Pharm Biol Sci Arch. 2011;2(4):1282–90.
Bertram Ulrike BR. In situ gelling, bioadhesive nasal inserts for extended drug delivery: in vitro characterization of a new nasal dosage form. Eur J Pharm Sci. 2006;27(1):62–71.
Madgulkar A, Kadam S, Pokharkar V. Studies on formulation development of mucoadhesive sustained release itraconazole tablet using response surface methodology. AAPS Pharm SciTech. 2008;9(3):998–1005.
Cook SL, Woods S, Methven L, Parker JK, Khutoryanskiy VV. Mucoadhesive polysaccharides modulate sodium retention, release and taste perception. Food Chem. 2018;1(240):482–9.
Madsen F, Eberth K, Smart JD. A rheological examination of the mucoadhesive/mucus interaction: the effect of mucoadhesive type and concentration. Journal of Controlled Release: official journal of the Controlled Release Society. 1998;50(1):167–78.
Shirizadeh B, Maghsoodi M, Alami-Milani M, Salatin S, Jelvehgari M. Tailored hydrogel microbeads of sodium carboxymethylcellulose as a carrier to deliver mefenamic acid: transmucosal administration. J Nat Pharm Prod. 2017;12(4):1–10.
Akbari J, Saeedi M, Morteza-Semnani K, Zarrabi B, Rostamkalaei SS, Kelidari HR. The effect of Plantago major seed mucilage combined with carbopol on the release profile and bioadhesive properties of propranolol HCl buccoadhesive tablets. Pharm Biomed Res. 2016;2(2):84–100.
Sasidhar RLC, Vidyadhara S, Maheswari GV, Deepti B, Srinivasa BP. Solubility and dissolution rate enhancement of olmesartan medoxomil by complexation and development of mouth dissolving tablets. Adv Biol Res. 2013;7(2):32–41.
Tile MK, Pawar AY. Solubility and dissolution rate enhancement of olmesartan medoxomil by chitosan base co-crystal approach. Int J Pharma Bio Sci. 2015;5(2):400–11.
Abdelkader H, Abdalla OY, Salem H. Formulation ofcontrolled-release baclofen matrix tablets: influence of some hydrophilic polymers on the release rate and in-vitro evaluation. AAPS Pharm Sci Tech. 2007;8(4):156–66.
Saha AK, Ray SD. Effect of cross-linked biodegradable polymers on sustained release of sodium diclofenac-loaded microspheres. Braz J Pharm Sci. 2013;49(4):874–88.
Yadav VK, Gupta AB, Kumar R, Yadav JS, Kumar B. Mucoadhesive polymers: means of improving the mucoadhesive properties of drug delivery system. J Chem Pharm Res. 2010;2(5):418–32.
Agarwal S, Murthy RSR. Effect of different polymer concentration on drug release rate and physicochemical properties of mucoadhesive gastroretentive tablets. Indian J Pharm Sci. 2015;77(6):705–14.
Alhalmil A, Altowairi M, Saeed O, Alzubaidi N, Almoiliqy M, Abdulmalik W. Sustained release matrix system: an overview. World J Pharm Pharm Sci. 2018;7(6):1470–86.
Yvonne P, Thomas R. Controlling drug delivery. 1st. London: Fastrack, Pharmaceutical Press; 2010.
Fahmy RH, Kassem MA. Enhancement of famotidine dissolution rate through liquisolid tablets formulation: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2008;69(3):993–1003.
Charman SA, Charman WN. Oral modified-release delivery systems. In: Modified-release drug delivery technology. California: Marcel Dekker Inc; 2003. p. 1–7.
Zhang X, Xing H, Zhao Y, Ma Z. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics. 2018;10(24):1–33.
Rodriguez C, Bruneau N, Barra J, Alfonso D, Doelker E, Wise D. Drug release from swelling-controlled systems. 2000.
Vigoreaux V, Ghaly ES. Fickian and relaxational contribution quantification of drug release in a swellable hydrophillic polymer matrix. Drug Dev Ind Pharm. 1994;20(16):2519–26.
Farid RM, Etman MA, Nada AH, Ebian AR. Formulation and in vitro evaluation of salbutamol sulphate in situ gelling nasal inserts. AAPS Pharm SciTech. 2013;14(2):712–8.
Prajapati ST, Joshi HA, Pate CN. Preparation and characterization of self-microemulsifying drug delivery system of olmesartan medoxomil for bioavailability improvement. J Pharm. 2013:1–9.
Aspden T, Illum L, Skaugrud Ø. Chitosan as a nasal delivery system: evaluation of insulin absorption enhancement and effect on nasal membrane integrity using rat models. Eur J Pharm Sci. 1996;4(1):23–31.
Fernandez-Urrusuno R, Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm Res. 1999;16(10):1576–81.
Oechslein CR, Fricker G, Kissel T. Nasal delivery of octreotide: absorption enhancement by particulate carrier systems. Int J Pharm. 1996;139(1):25–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study design was approved by the Cairo University Research Ethics Committee (serial number of the protocol: PI 1189) and conducted with respect to the world medical association’s code of ethics of Declaration of Helsinki 2013.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rashad, A.A., Nageeb El-Helaly, S., Abd El Rehim, R.T. et al. Chronological Delivery of Antihypertensive Drugs in Bilayered Core-in-Cup Buccoadhesive Tablets: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 21, 21 (2020). https://doi.org/10.1208/s12249-019-1575-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1575-9