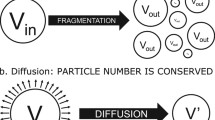
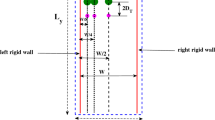
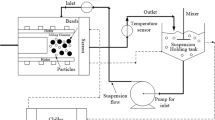
Abstract
Viscosity, influenced by medium composition, will affect the hydrodynamics of a dissolution system. Dissolution simulation methods are valuable tools to explore mechanistic dissolution effects, with an understanding of limitations of any simulation method essential to its appropriate use. The aims of this paper were a) to explore, using dissolution simulation, the effects of slightly viscous media on particulate dissolution and b) to illustrate approaches to, and limitations of, the dissolution simulations. A lumped parameter fluid dynamics dissolution simulation model (SIMDISSO™) was used to simulate particulate (20 and 200 μm diameter) dissolution in media with viscosity at 37 °C of water (0.7 mPa.s), milk (1.4 mPa.s) and a nutrient drink (12.3 mPa.s). Effects of flow rate, modality (constant vs pulsing), viscosity and gravitational and particle motion/sedimentation effects on simulated dissolution were explored, in the flow through and paddle apparatuses as appropriate. Shadowgraph imaging (SGI) was used to visualise particle suspension behaviour. Flow rate, hydrodynamic viscous effects and disabling particle motion and gravitational effects affected simulated dissolution of larger particles. SGI imaging revealed retention of particles in suspension in 1.4 mPa.s medium, which sedimented in water. The effect of diffusion adjusted for viscosity was significant for both particle sizes. The limitations of this 1D simulation approach would be greater for larger particles in low velocity regions of the paddle apparatus. Even slightly viscous media can affect dissolution of larger particles with dissolution simulation affording insight into the mechanisms involved, provided the assumptions and limitations of the simulation approach are clarified and understood.










Similar content being viewed by others
References
Todaro V, Persoons T, Grove G, Healy AM, D’Arcy DM. Characterization and simulation of hydrodynamics in the paddle, basket and flow-through dissolution testing apparatuses-a review. Dissolut Technol. 2017;24(3):24–36.
Markopoulos C, Andreas CJ, Vertzoni M, Dressman J, Reppas C. In-vitro simulation of luminal conditions for evaluation of performance of oral drug products: choosing the appropriate test media. Eur J Pharm Biopharm. 2015;93:173–82.
Maharaj AR, Edginton AN, Fotaki N. Assessment of age-related changes in pediatric gastrointestinal solubility. Pharm Res. 2016;33(1):52–71. https://doi.org/10.1007/s11095-015-1762-7.
Kostewicz ES, Abrahamsson B, Brewster M, Brouwers J, Butler J, Carlert S, et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:342–66. https://doi.org/10.1016/j.ejps.2013.08.024.
McAllister M. Dynamic dissolution: a step closer to predictive dissolution testing? Mol Pharm. 2010;7(5):1374–87. https://doi.org/10.1021/mp1001203.
Pedersen PB, Vilmann P, Bar-Shalom D, Müllertz A, Baldursdottir S. Characterization of fasted human gastric fluid for relevant rheological parameters and gastric lipase activities. Eur J Pharm Biopharm. 2013;85(3):958–65.
Reppas C, Karatza E, Goumas C, Markopoulos C, Vertzoni M. Characterization of contents of distal ileum and cecum to which drugs/drug products are exposed during bioavailability/bioequivalence studies in healthy adults. Pharm Res. 2015;32(10):3338–49. https://doi.org/10.1007/s11095-015-1710-6.
Jantratid E, Janssen N, Reppas C, Dressman JB. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm Res. 2008;25(7):1663–76. https://doi.org/10.1007/s11095-008-9569-4.
Garbacz G, Cadé D, Benameur H, Weitschies W. Bio-relevant dissolution testing of hard capsules prepared from different shell materials using the dynamic open flow through test apparatus. Eur J Pharm Sci. 2014;57:264–72.
Sun W, Houghton LA, Read N, Grundy D, Johnson A. Effect of meal temperature on gastric emptying of liquids in man. Gut. 1988;29(3):302–5.
Koziolek M, Grimm M, Becker D, Iordanov V, Zou H, Shimizu J, et al. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the Intellicap® system. J Pharm Sci. 2015;104(9):2855–63.
Schiller C, Frohlich CP, Giessmann T, Siegmund W, Monnikes H, Hosten N, et al. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2005;22(10):971–9. https://doi.org/10.1111/j.1365-2036.2005.02683.x.
Koziolek M, Schneider F, Grimm M, Modebeta C, Seekamp A, Roustom T, et al. Intragastric pH and pressure profiles after intake of the high-caloric, high-fat meal as used for food effect studies. J Control Release. 2015;220(Pt A):71–8. https://doi.org/10.1016/j.jconrel.2015.10.022.
Perivilli S, Kakhi M, Stippler E. Computational fluid dynamics simulation of hydrodynamics in USP apparatus 3-the influence of dip rate. Pharm Res. 2015;32(4):1304–15. https://doi.org/10.1007/s11095-014-1534-9.
Perivilli S, Prevost R, Stippler E. Velocity field visualization in USP dissolution apparatus 3 using particle image velocimetry. Pharm Res. 2017;34(6):1330–7. https://doi.org/10.1007/s11095-017-2151-1.
Pal A, Indireshkumar K, Schwizer W, Abrahamsson B, Fried M, Brasseur JG. Gastric flow and mixing studied using computer simulation. Proc R Soc B. 2004;271(1557):2587–94. https://doi.org/10.1098/rspb.2004.2886.
Du P, O'Grady G, Gao J, Sathar S, Cheng LK. Toward the virtual stomach: progress in multiscale modeling of gastric electrophysiology and motility. Wiley Interdiscip Rev Syst Biol Med. 2013;5(4):481–93. https://doi.org/10.1002/wsbm.1218.
Du P, Paskaranandavadivel N, Angeli TR, Cheng LK, O'Grady G. The virtual intestine: in silico modeling of small intestinal electrophysiology and motility and the applications. Wiley Interdiscip Rev Syst Biol Med. 2016;8(1):69–85. https://doi.org/10.1002/wsbm.1324.
Ferrua MJ, Singh RP. Modeling the fluid dynamics in a human stomach to gain insight of food digestion. J Food Sci. 2010;75(7):R151–62. https://doi.org/10.1111/j.1750-3841.2010.01748.x.
Imai Y, Kobayashi I, Ishida S, Ishikawa T, Buist M, Yamaguchi T. Antral recirculation in the stomach during gastric mixing. Am J Phys. 2013;304(5):G536–42. https://doi.org/10.1152/ajpgi.00350.2012.
Klein S, Butler J, Hempenstall JM, Reppas C, Dressman JB. Media to simulate the postprandial stomach I. Matching the physicochemical characteristics of standard breakfasts. J Pharm Pharmacol. 2004;56(5):605–10. https://doi.org/10.1211/0022357023367.
Radwan A, Wagner M, Amidon GL, Langguth P. Bio-predictive tablet disintegration: effect of water diffusivity, fluid flow, food composition and test conditions. Eur J Pharm Sci. 2014;57:273–9.
Korson L, Drost-Hansen W, Millero FJ. Viscosity of water at various temperatures. J Phys Chem. 1969;73(1):34–9.
Klein S. The use of biorelevant dissolution media to forecast the in vivo performance of a drug. AAPS J. 2010;12(3):397–406.
Sugano K. Theoretical comparison of hydrodynamic diffusion layer models used for dissolution simulation in drug discovery and development. Int J Pharm. 2008;363(1–2):73–7. https://doi.org/10.1016/j.ijpharm.2008.07.002.
D’Arcy DM, Persoons T. Mechanistic modelling and mechanistic monitoring: simulation and shadowgraph imaging of particulate dissolution in the flow-through apparatus. J Pharm Sci. 2011;100(3):1102–15. https://doi.org/10.1002/jps.22337.
United States Pharmacopoeia 41/National Formulary 36. Rockwell, MD, USA: The United States Pharmacopeial Convention; 2018.
Serrano DR, Persoons T, D'Arcy DM, Galiana C, Dea-Ayuela MA, Healy AM. Modelling and shadowgraph imaging of cocrystal dissolution and assessment of in vitro antimicrobial activity for sulfadimidine/4-aminosalicylic acid cocrystals. Eur J Pharm Sci. 2016;89:125–36. https://doi.org/10.1016/j.ejps.2016.04.030.
Bird R, Stewart W, Lightfoot E. Transport phenomena. 2nd ed. New York: Wiley; 2002.
Kovacevic I, Parojcic J, Homsek I, Tubic-Grozdanis M, Langguth P. Justification of biowaiver for carbamazepine, a low soluble high permeable compound, in solid dosage forms based on IVIVC and gastrointestinal simulation. Mol Pharm. 2009;6(1):40–7. https://doi.org/10.1021/mp800128y.
Lee H, Park SA, Sah H. Surfactant effects upon dissolution patterns of carbamazepine immediate release tablet. Arch Pharm Res. 2005;28(1):120–6.
Diebold SM, Dressman JB. Hydrodynamik kompendialer Lösungsgeschwindigkeits-Testapparaturen Paddle und Basket. Pharm Ind. 2001;63(1):94–104.
Diebold SM. Hydrodynamik und Losungsgeschwindigkeit-Untersuchungen zum Einfluss der Hydrodynamik auf die Losungsgeschindigkeit schwer wasserloslicher Arzneistoffe. Frankfurt am Main. Germany: Johann Wolfgang Goethe Universitat; 2000.
D’Arcy DM, Corrigan OI, Healy AM. Hydrodynamic simulation (computational fluid dynamics) of asymmetrically positioned tablets in the paddle dissolution apparatus: impact on dissolution rate and variability. J Pharm Pharmacol. 2005;57:1243–50.
D’Arcy DM. Use of computational fluid dynamics to investigate the relationship between hydrodynamics and rates of dissolution. Trinity College Dublin: Dublin; 2007.
Anwar S, Fell J, Dickinson P. An investigation of the disintegration of tablets in biorelevant media. Int J Pharm. 2005;290(1–2):121–7.
Cvijić S, Parojčić J, Langguth P. Viscosity-mediated negative food effect on oral absorption of poorly-permeable drugs with an absorption window in the proximal intestine: in vitro experimental simulation and computational verification. Eur J Pharm Sci. 2014;61:40–53.
Radwan A, Ebert S, Amar A, Münnemann K, Wagner M, Amidon GL, et al. Mechanistic understanding of food effects: water diffusivity in gastrointestinal tract is an important parameter for the prediction of disintegration of solid oral dosage forms. Mol Pharm. 2013;10(6):2283–90.
Radwan A, Amidon GL, Langguth P. Mechanistic investigation of food effect on disintegration and dissolution of BCS class III compound solid formulations: the importance of viscosity. Biopharm Drug Dispos. 2012;33(7):403–16. https://doi.org/10.1002/bdd.1798.
Braun RJ, Parrott EL. Influence of viscosity and solubilization on dissolution rate. J Pharm Sci. 1972;61(2):175–8.
Sarisuta N, Parrott EL. Relationship of dissolution rate to viscosity of polymeric solutions. J Pharm Sci. 1982;71(12):1375–80.
Nelson K, Shah A. Mass transport in dissolution kinetics I: convective diffusion to assess the role of fluid viscosity under forced flow conditions. J Pharm Sci. 1987;76(10):799–802.
Shah A, Nelson K. Mass transport in dissolution kinetics II: convective diffusion to assess role of viscosity under conditions of gravitational flow. J Pharm Sci. 1987;76(12):910–3.
Zarmpi P, Flanagan T, Meehan E, Mann J, Fotaki N. Biopharmaceutical understanding of excipient variability: effect of HPMC level and viscosity type on drug solubility. AAPS Annual Meeting; San Diego, USA: http://abstracts.aaps.org/published/; 2017.
Higuchi M, Yoshihashi Y, Tarada K, Sugano K. Minimum rotation speed to prevent coning phenomena in compendium paddle dissolution apparatus. Eur J Pharm Sci. 2014;65:74–8. https://doi.org/10.1016/j.ejps.2014.09.010.
Higuchi M, Terada K, Sugano K. Coning phenomena under laminar flow. Eur J Pharm Sci. 2015;80:53–5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Sandra Klein
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
D’Arcy, D.M., Persoons, T. Understanding the Potential for Dissolution Simulation to Explore the Effects of Medium Viscosity on Particulate Dissolution. AAPS PharmSciTech 20, 47 (2019). https://doi.org/10.1208/s12249-018-1260-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-018-1260-4