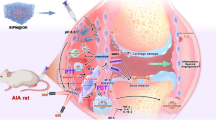
Abstract
Aspasomes of methotrexate with antioxidant, ascorbyl palmitate, were developed and optimized using factorial design by varying parameters such as lipid molar ratio, drug to lipid molar ratio, and type of hydration buffer for transdermal delivery for disease modifying activity in rheumatoid arthritis (RA). Aspasomes were characterized by drug-excipients interaction, particle size analysis, determination of zeta potential, entrapment efficiency, and surface properties. The best formulation was loaded into hydrogel for evaluation of in vitro drug release and tested in vivo against adjuvant induced arthritis model in wistar rats, by assessing various physiological, biochemical, hematological, and histopathological parameters. Optimized aspasome formulation exhibited smooth surface with particle size 386.8 nm, high drug loading (19.41%), negative surface potential, and controlled drug release in vitro over 24 h with a steady permeation rate. Transdermal application of methotrexate-loaded aspasome hydrogel for 12 days reduced rat paw diameter (21.25%), SGOT (40.43%), SGPT (54.75%), TNFα (33.99%), IL β (34.79%), cartilage damage (84.41%), inflammation (82.37%), panus formation (84.38%), and bone resorption (80.52%) as compared to arthritic control rats. Free methotrexate-treated group showed intermediate effects. However, drug-free aspasome treatment did not show any effect. The experimental results indicate a positive outcome in development of drug-loaded therapeutically active carrier system which presents a non-invasive controlled release transdermal formulation with good drug loading, drug permeation rate, and having better disease modifications against RA than the free drug, thereby providing a more attractive therapeutic strategy for rheumatoid disease management.





Similar content being viewed by others
References
Kraft JC, Freeling JP, Wang Z, Ho RJ. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci. 2014;103(1):29–52. https://doi.org/10.1002/jps.23773.
Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153(3):198–205. https://doi.org/10.1016/j.jconrel.2011.06.001.
Gopinath D, Ravi D, Rao BR, Apte SS, Renuka D, Rambhau D. Ascorbylpalmitate vesicles (Aspasomes): formation, characterization and applications. Int J Pharm. 2004;271(1–2):95–113. https://doi.org/10.1016/j.ijpharm.2003.10.032.
Jukanti R, Gopinath D, Apte SS, Rambhau D. Biodistribution of ascorbylpalmitate loaded doxorubicin pegylated liposomes in solid tumor bearing mice. J Microencapsul. 2011;28(2):142–9. https://doi.org/10.3109/02652048.2010.542496.
Lee S, Lee J, Choi YW. Skin permeation enhancement of ascorbyl palmitate by liposomal hydrogel (lipogel) formulation and electrical assistance. Biol Pharm Bull. 2007;30(2):393–6. https://doi.org/10.1248/bpb.30.393.
Moribe K, Limwikrant W, Higashi K, Yamamoto K. Drug nanoparticle formulation using ascorbic acid derivatives. J Drug Deliv. 2011;2011:1–9. https://doi.org/10.1155/2011/138929.
Karande P, Mitragotri S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim Biophys Acta. 2009;1788(11):2362–73. https://doi.org/10.1016/j.bbamem.2009.08.015.
Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev. 2005;57(2):163–72. https://doi.org/10.1124/pr.57.2.3.
Gerards AH, de Lathouder S, de Groot ER, Dijkmans BAC, Aarden LA. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers, patients with rheumatoid arthritis. Rheumatology. 2003;42(10):1189–96. https://doi.org/10.1093/rheumatology/keg323.
Gottschalk O, Metz P, Dao Trong ML, Altenberger S, Jansson V, Mutschler W, et al. Therapeutic effect of methotrexate encapsulated in cationic liposomes (EndoMTX) in comparison to free methotrexate in an antigen-induced arthritis study in vivo. Scand J Rheumatol. 2015;44(6):456–63. https://doi.org/10.3109/03009742.2015.1030448.
Garg NK, Singh B, Tyagi RK, Sharma G, Katare OP. Effective transdermal delivery of methotrexate through nanostructured lipid carriers in an experimentally induced arthritis model. Colloids Surf B Biointerfaces. 2016;147:17–24. https://doi.org/10.1016/j.colsurfb.2016.07.046.
Prabhu P, Shetty R, Koland M, Vijayanarayana K, Vijayalakshmi KK, Nairy MH, et al. Investigation of nano lipid vesicles of methotrexate for anti-rheumatoid activity. Int J Nanomedicine. 2012;7:177–86. https://doi.org/10.2147/IJN.S25310.
Roy T, Ghosh S. Animal models of rheumatoid arthritis: correlation and usefulness with human rheumatoid arthritis. Indo Amer J Pharm Res. 2013;3:6131–42.
Chang RK, Raw A, Lionberger R, Yu L. Generic development of topical dermatologic products: formulation development, process development, and testing of topical dermatologic products. AAPS J. 2013;15(1):41–52. https://doi.org/10.1208/s12248-012-9411-0.
Grijalvo S, Mayr J, Eritja R, Díaz DD. Biodegradable liposome-encapsulated hydrogels for biomedical applications: a marriage of convenience. Biomater Sci. 2016;4(4):555–74. https://doi.org/10.1039/C5BM00481K.
De P, Damodharan N, Mallick S, Mukherjee B. Development and evaluation of nefopam transdermal matrix patch system in human volunteers. PDA J Pharm Sci Technol. 2009;63(6):537–46.
Pattnaik G, Sinha B, Mukherjee B, Ghosh S, Basak S, Mondal S, et al. Submicron-size biodegradable polymer-based didanosine particles for treating HIV at early stage: an in vitro study. J Microencapsul. 2012;29(7):666–76. https://doi.org/10.3109/02652048.2012.680509.
Ghosh S, Mondal L, Chakraborty S, Mukherjee B. Early stage HIV management and reduction of stavudine-induced hepatotoxicity in rats by experimentally developed biodegradable nanoparticles. AAPS PharmSciTech. 2017;18(3):697–709. https://doi.org/10.1208/s12249-016-0539-6.
Ng SF, Rouse J, Sanderson D, Eccleston GA. Comparative study of transmembrane diffusion and permeation of ibuprofen across synthetic membranes using Franz diffusion cells. Pharmaceutics. 2010;2(2):209–23. https://doi.org/10.3390/pharmaceutics2020209.
Sinicoa C, Manconia M, Peppib M, Laia F, Valentia D, Fadda AM. Liposomes as carriers for dermal delivery of tretinoin: in vitro evaluation of drug permeation and vesicle–skin interaction. J Control Release. 2005;103(1):123–36. https://doi.org/10.1016/j.jconrel.2004.11.020.
Takeuchi H, Mano Y, Terasaka S, Sakurai T, Furuya A, Urano H, et al. Usefulness of rat skin as a substitute for human skin in the in vitro skin permeation study. Exp Anim. 2011;60(4):373–84. https://doi.org/10.1538/expanim.60.373.
Roy T, Banerjee I, Ghosh S, Dhali RS, Pati AD, Tripathi SK. Effects of co-treatment with pioglitazone and methotrexate on experimentally induced rheumatoid arthritis in Wistar albino rats. Indian J Pharmacol. 2017;49(2):168–75. https://doi.org/10.4103/ijp.IJP_523_15.
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. https://doi.org/10.4103/0976-0105.177703.
Martínez A, Fernández-Arquero M, Pascual-Salcedo D, Conejero L, Alves H, Balsa A, et al. Primary association of tumour necrosis factor-region genetic markers with susceptibility to rheumatoid arthritis. Arthritis Rheum. 2000;43(6):1366–70. https://doi.org/10.1002/1529-0131(200006)43:6<1366::AID-ANR21>3.0.CO;2-S.
Thorbecke GJ, Shah R, Leu CH, Kuruvilla AP, Hardison AM, Palladino MA. Involvement of endogenous tumor necrosis factor alpha and transforming growth factor beta during induction of collagen type II arthritis in mice. Proc Natl Acad Sci U S A. 1992;89(16):7375–9. https://doi.org/10.1073/pnas.89.16.7375.
Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, Jain NK. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release. 2007;123(2):148–54. https://doi.org/10.1016/j.jconrel.2007.08.005.
Gosenca M, Obreza A, Pečar S, Gašperlin M. A new approach for increasing ascorbyl palmitate stability by addition of non-irritant co-antioxidant. AAPS PharmSciTech. 2010;11(3):1485–92. https://doi.org/10.1208/s12249-010-9507-8.
Andersen FA. Final report on the safety assessment of ascorbylpalmitate, ascorbyldipalmitate, ascorbyl stearate, erythorbic acid, and sodium erythorbate. Int J Toxicol. 1999;18(3):1–26.
Anderson M, Omri A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv. 2004;11(1):33–9. https://doi.org/10.1080/10717540490265243.
Kristl J, Volka B, Gasperlin M, Sentjurc M, Jurkovic P. Effect of colloidal carriers on ascorbylpalmitate stability. Eur J Pharm Sci. 2003;19(4):181–9. https://doi.org/10.1016/S0928-0987(03)00104-0.
Obeida MA, Khadraa I, Mullena A, Tatea RJ, Ferroa VA. The effects of hydration media on the characteristics of non-ionic surfactant vesicles (NISV) prepared by microfluidics. Int J Pharm. 2017;516(1–2):52–60. https://doi.org/10.1016/j.ijpharm.2016.11.015.
Ruckmani K, Sankar V. Formulation and optimization of zidovudine niosomes. AAPS PharmSciTech. 2010;11(3):1119–27. https://doi.org/10.1208/s12249-010-9480-2.
Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Ravi Kumar MNV. Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Control Release. 2006;113(3):189–207. https://doi.org/10.1016/j.jconrel.2006.04.015.
Chibowski E, Szcześ A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption. 2016;22(4):755–65. https://doi.org/10.1007/s10450-016-9767-z.
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. https://doi.org/10.1186/1556-276X-8-102.
Hoarea TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49(8):1993–2007. https://doi.org/10.1016/j.polymer.2008.01.027.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23.
Abd E, Yousef SA, Pastore MN, Telaprolu K, Mohammed YH, Namjoshi S, et al. Skin models for the testing of transdermal drugs. Clin Pharmacol. 2016;8:163–76. https://doi.org/10.2147/CPAA.S64788.
Chung CP, Avalos I, Raggi P, Stein CM. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin Rheumatol. 2007;26(8):1228–33. https://doi.org/10.1007/s10067-007-0548-7.
Snekhalatha U, Anburajan M, Venkatraman B, Menaka M. Evaluation of complete Freund’s adjuvant-induced arthritis in a Wistar rat model: comparison of thermography and histopathology. Z Rheumatol. 2013;72(4):375–82. https://doi.org/10.1007/s00393-012-1083-8.
Asquith DL, Miller AM, McInnes IB, Liew FY. Animal models of rheumatoid arthritis. Eur J Immunol. 2009;39(8):2040–4. https://doi.org/10.1002/eji.200939578.
Goodson T, Morgan SL, Carlee JR, Baggott JE. The energy cost of adjuvant-induced arthritis in rats. Arthritis Rheum. 2003;48(10):2979–82. https://doi.org/10.1002/art.11274.
Somasundaran S, Sadique J, Subramoniam A. In vitro absorption of [14C] leucine during inflammation and the effect of anti-inflammatory drugs in the jejunum of rats. Biochem Med. 1983;29(2):259–64. https://doi.org/10.1016/0006-2944(83)90046-7.
Stagakis I, Bertsias G, Karvounaris S, Kavousanaki M, Virla D, Raptopoulou A, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14(3):R141. https://doi.org/10.1186/ar3874.
Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation-mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32(8):1771–6. https://doi.org/10.1161/ATVBAHA.111.241869.
Feldmann M, Brennan FM, Foxwell BM, Maini RN. The role of TNF alpha and IL-1 in rheumatoid arthritis. Curr Dir Autoimmun. 2001;3:188–99.
McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–42. https://doi.org/10.1038/nri2094.
Kinne RW, Brauer R, Stuhlmuller B, Kinne RW, Bräuer R, Stuhlmüller B, et al. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2(3):189–202. https://doi.org/10.1186/ar86.
Karie S, Gandjbakhch F, Janus N, Launay-Vacher V, Rozenberg S, Mai Ba CU, et al. Kidney disease in RA patients: prevalence and implication on RA-related drugs management: the MATRIX study. Rheumatology (Oxford). 2008;47(3):350–4. https://doi.org/10.1093/rheumatology/kem370.
Selmi C, De Santis M, Gershwin ME. Liver involvement in subjects with rheumatic disease. Arthritis Res Ther. 2011;13(3):226. https://doi.org/10.1186/ar3319.
Rajkapoor B, Ravichandran V, Gobinath M, Anbu J, Harikrishnan N, Sumithra M, et al. Effect of Bauhinia variegata on complete Freund’s adjuvant induced arthritis in rats. J Pharmacol Toxicol. 2007;2(5):465–72.
Ormseth MJ, Oeser AM, Cunningham A, Bian A, Shintani A, Solus J, et al. Peroxisome proliferator-activated receptor gamma agonist effect on rheumatoid arthritis: a randomized controlled trial. Arthritis Res Ther. 2013;15(5):R110. https://doi.org/10.1186/ar4290.
Trotta M, Peira E, Carlotti ME, Gallarate M. Deformable liposomes for dermal administration of methotrexate. Int J Pharm. 2004;270(1-2):119–25. https://doi.org/10.1016/j.ijpharm.2003.10.006.
Alvarez-Figueroa MJ, Delgado-Charro MB, Blanco-Mèndez J. Passive and iontophoretic transdermal penetration of methotrexate. Int J Pharm. 2001;212(1):101–7. https://doi.org/10.1016/S0378-5173(00)00599-8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ghosh, S., Mukherjee, B., Chaudhuri, S. et al. Methotrexate Aspasomes Against Rheumatoid Arthritis: Optimized Hydrogel Loaded Liposomal Formulation with In Vivo Evaluation in Wistar Rats. AAPS PharmSciTech 19, 1320–1336 (2018). https://doi.org/10.1208/s12249-017-0939-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0939-2