Abstract
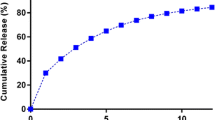
The purpose of the present study was to develop a rebamipide (RBM) gastro-retentive (GR) tablet by implementing quality by design (QbD). RBM GR tablets were prepared using a sublimation method. Quality target product profile (QTPP) and critical quality attributes (CQAs) of the RBM GR tablets were defined according to the preliminary studies. Factors affecting the CQAs were prioritized using failure mode and effects analysis (FMEA). Design space and optimum formulation were established through a mixture design. The validity of the design space was confirmed using runs within the area. The QTPP of the RBM GR tablets was the orally administered GR tablet containing 300 mg of RBM taken once daily. Based on the QTPP, dissolution rate, tablet friability, and floating property were chosen as CQAs. According to the risk assessment, the amount of sustained-release agent, sublimating material, and diluent showed high-risk priority number (RPN) values above 40. Based on the RPN, these factors were further investigated using mixture design methodology. Design space of formulations was depicted as an overlaid contour plot and the optimum formulation to satisfy the desired responses was obtained by determining the expected value of each response. The similarity factor (f2) of the release profile between predicted response and experimental response was 89.463, suggesting that two release profiles are similar. The validity of the design space was also confirmed. Consequently, we were able to develop the RBM GR tablets by implementing the QbD concept. These results provide useful information for development of tablet formulations using the QbD.




Similar content being viewed by others
References
Yamasaki K, Kanbe T, Chijiwa T, Ishiyama H, Morita S. Gastric mucosal protection by OPC-12759, a novel antiulcer compound, in the rat. Eur J Pharmacol. 1987;142(1):23–9.
Tarnawski AS. 15th anniversary of rebamipide: looking ahead to the new mechanisms and new applications. Dig Dis Sci. 2005;50(1):S3–S11.
Ogino K, Hobara T, Ishiyama H, Yamasaki K, Kobayashi H, Izumi Y, et al. Antiulcer mechanism of action of rebamipide, a novel antiulcer compound, on diethyldithiocarbamate-induced antral gastric ulcers in rats. Eur J Pharmacol. 1992;212(1):9–13.
Tarnawski A, Chai J, Pai R, Chiou S-K. Rebamipide activates genes encoding angiogenic growth factors and Cox2 and stimulates angiogenesis: a key to its ulcer healing action? Dig Dis Sci. 2004;49(2):202–9.
Murata H, Yabe Y, Tsuji S, Tsujii M, Fu HY, Asahi K, et al. Gastroprotective agent rebamipide induces cyclooxygenase-2 (COX-2) in gastric epithelial cells. Dig Dis Sci. 2005;50(1):S70–S5.
Ho L, Müller R, Römer M, Gordon K, Heinämäki J, Kleinebudde P, et al. Analysis of sustained-release tablet film coats using terahertz pulsed imaging. J Control Release. 2007;119(3):253–61.
Yamamoto T, Isono A, Mishina Y, Ebato T, Shirai T, Nakayama S, et al. Gastroduodenal mucosal injury in patients taking low-dose aspirin and the role of gastric mucoprotective drugs: possible effect of rebamipide. J Clin Biochem Nutr. 2010;47(1):27–31.
Araki H, Kato T, Onogi F, Ibuka T, Sugiyama A, Nakanishi T, et al. Combination of proton pump inhibitor and rebamipide, a free radical scavenger, promotes artificial ulcer healing after endoscopic submucosal dissection with dissection size >40 mm. J Clin Biochem Nutr. 2012;51(3):185–8.
Streubel A, Siepmann J, Bodmeier R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr Opin Pharmacol. 2006;6(5):501–8.
Talukder R, Fassihi R. Gastroretentive delivery systems: a mini review. Drug Dev Ind Pharm. 2004;30(10):1019–28.
Kim J-Y, Seo J-W, Rhee Y-S, Park C-W, Park E-S. Freeze-dried highly porous matrix as a new gastroretentive dosage form for ecabet sodium: in vitro and in vivo characterizations. J Pharm Sci. 2014;103(1):262–73.
Oh TO, Kim JY, Ha JM, Chi SC, Rhee YS, Park CW, et al. Preparation of highly porous gastroretentive metformin tablets using a sublimation method. Eur J Pharm Biopharm. 2013;83(3):460–7.
Lopes CM, Bettencourt C, Rossi A, Buttini F, Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int J Pharm. 2016;510(1):144–58.
Chen YC, Ho HO, Lee TY, Sheu MT. Physical characterizations and sustained release profiling of gastroretentive drug delivery systems with improved floating and swelling capabilities. Int J Pharm. 2013;441(1–2):162–9.
Darandale SS, Vavia PR. Design of a gastroretentive mucoadhesive dosage form of furosemide for controlled release. Acta Pharm Sin B. 2012;2(5):509–17.
Pund S, Joshi A, Vasu K, Nivsarkar M, Shishoo C. Gastroretentive delivery of rifampicin: in vitro mucoadhesion and in vivo gamma scintigraphy. Int J Pharm. 2011;411(1–2):106–12.
Jagdale S, Kurhe P, Kuchekar B, Chabukswar A. Application of design of experiments to optimizing novel gastroretentive drug delivery of simvastatin. Curr Drug Deliv. 2013;10(5):527–41.
Thakar K, Joshi G, Sawant KK. Bioavailability enhancement of baclofen by gastroretentive floating formulation: statistical optimization, in vitro and in vivo pharmacokinetic studies. Drug Dev Ind Pharm. 2013;39(6):880–8.
Zidan AS, Sammour OA, Hammad MA, Megrab NA, Habib MJ, Khan MA. Quality by design: understanding the formulation variables of a cyclosporine A self-nanoemulsified drug delivery systems by Box–Behnken design and desirability function. Int J Pharm. 2007;332(1–2):55–63.
Ibrahim HM, Ahmed TA, Hussain MD, Rahman Z, Samy AM, Kaseem AA, et al. Development of meloxicam in situ implant formulation by quality by design principle. Drug Dev Ind Pharm. 2014;40(1):66–73.
Shastri PN, Ubale RV, D’Souza MJ. Implementation of mixture design for formulation of albumin containing enteric-coated spray-dried microparticles. Drug Dev Ind Pharm. 2013;39(2):164–75.
Huang C-T, Tsai M-J, Lin Y-H, Fu Y-S, Huang Y-B, Tsai Y-H, et al. Effect of microemulsions on transdermal delivery of citalopram: optimization studies using mixture design and response surface methodology. Int J Nanomedicine. 2013;8:2295–304.
El-Dahmy RM, Elsayed I, Elshafeey AH, Gawad NAAE, El-Gazayerly ON. Optimization of long circulating mixed polymeric micelles containing vinpocetine using simple lattice mixture design, in vitro and in vivo characterization. Int J Pharm. 2014;477(1–2):39–46.
ICH Expert Working Group. Pharmaceutical development Q8(R2). The International Council for Harmonisation. 2009. https://www.aaps.org/uploadedFiles/Content/Publications/Journals/AAPSPT%20Instructions%20for%20Authors_Updated%209.19.16(1).pdf.
Lionberger RA, Lee SL, Lee L, Raw A, Lawrence XY. Quality by design: concepts for ANDAs. AAPS J. 2008;10(2):268–76.
Lawrence XY. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25(4):781–91.
Fahmy R, Kona R, Dandu R, Xie W, Claycamp G, Hoag SW. Quality by design I: application of failure mode effect analysis (FMEA) and Plackett–Burman design of experiments in the identification of “main factors” in the formulation and process design space for roller-compacted ciprofloxacin hydrochloride immediate-release tablets. AAPS PharmSciTech. 2012;13(4):1243–54.
Vora C, Patadia R, Mittal K, Mashru R. Risk based approach for design and optimization of stomach specific delivery of rifampicin. Int J Pharm. 2013;455(1–2):169–81.
Hwang K-M, Cho C-H, Tung N-T, Kim J-Y, Rhee Y-S, Park E-S. Release kinetics of highly porous floating tablets containing cilostazol. Eur J Pharm Biopharm. 2017;115:39–51.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (grant number NRF-2016R1A2B4007101).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ha, JM., Seo, JW., Kim, SH. et al. Implementation of Quality by Design for Formulation of Rebamipide Gastro-retentive Tablet. AAPS PharmSciTech 18, 3129–3139 (2017). https://doi.org/10.1208/s12249-017-0797-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0797-y