Abstract
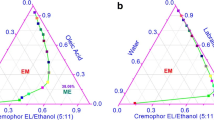
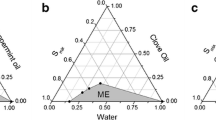
In this study, non-aqueous microemulsions were developed because of the challenges associated with finding pharmaceutically acceptable solvents for topical delivery of drugs sparingly soluble in water. The formulation irritation potential and ability to modulate the penetration of lipophilic compounds (progesterone, α-tocopherol, and lycopene) of interest for topical treatment/prevention of skin disorders were evaluated and compared to solutions and aqueous microemulsions of similar composition. The microemulsions (ME) were developed with BRIJ, vitamin E-TPGS, and ethanol as surfactant-co-surfactant blend and tributyrin, isopropyl myristate, and oleic acid as oil phase. As polar phase, propylene glycol (MEPG) or water (MEW) was used (26% w/w). The microemulsions were isotropic and based on viscosity and conductivity assessment, bicontinuous. Compared to drug solutions in lipophilic vehicles, MEPG improved drug delivery into viable skin layers by 2.5–38-fold; the magnitude of penetration enhancement mediated by MEPG into viable skin increased with drug lipophilicity, even though the absolute amount of drug delivered decreased. Delivery of progesterone and tocopherol, but not lycopene (the most lipophilic compound), increased up to 2.5-fold with MEW, and higher amounts of these two drugs were released from MEW (2–2.5-fold). Both microemulsions were considered safe for topical application, but MEPG-mediated decrease in the viability of reconstructed epidermis was more pronounced, suggesting its higher potential for irritation. We conclude that MEPG is a safe and suitable nanocarrier to deliver a variety of lipophilic drugs into viable skin layers, but the use of MEW might be more advantageous for drugs in the lower range of lipophilicity.






Similar content being viewed by others
References
Moniruzzaman M, Tamura M, Tahara Y, Kamiya N, Goto M. Ionic liquid-in-oil microemulsion as a potential carrier of sparingly soluble drug: characterization and cytotoxicity evaluation. Int J Pharm. 2010;400(1–2):243–50.
Lin CC, Yang CH, Chang NF, Wu PS, Chen YS, Lee SM, et al. Study on the stability of deoxyArbutin in an anhydrous emulsion system. Int J Mol Sci. 2011;12(9):5946–54.
Moser K, Kriwet K, Naik A, Kalia YN, Guy RH. Passive skin penetration enhancement and its quantification in vitro. Eur J Pharm Biopharm. 2001;52(2):103–12.
Cichewicz A, Pacleb C, Connors A, Hass MA, Lopes LB. Cutaneous delivery of alpha-tocopherol and lipoic acid using microemulsions: influence of composition and charge. J Pharm Pharmacol. 2013;65(6):817–26.
Lopes LB. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics. 2014;6(1):52–77.
Santos P, Watkinson AC, Hadgraft J, Lane ME. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol Physiol. 2008;21(5):246–59.
Zhang J, Michniak-Kohn B. Investigation of microemulsion microstructures and their relationship to transdermal permeation of model drugs: ketoprofen, lidocaine, and caffeine. Int J Pharm. 2011;421(1):34–44.
Pepe D, McCall M, Zheng H, Lopes LB. Protein transduction domain-containing microemulsions as cutaneous delivery systems for an anticancer agent. J Pharm Sci. 2013;102(5):1476–87.
Thomas S, Vieira CS, Hass MA, Lopes LB. Stability, cutaneous delivery, and antioxidant potential of a lipoic acid and alpha-tocopherol codrug incorporated in microemulsions. J Pharm Sci. 2014;103(8):2530–8.
Lv FF, Zheng LQ, Tung CH. Phase behavior of the microemulsions and the stability of the chloramphenicol in the microemulsion-based ocular drug delivery system. Int J Pharm. 2005;301(1–2):237–46.
Hamill RD, Petersen RV. Effect of surfactant concentration on the interfacial viscosity of a nonaqueous system. J Pharm Sci. 1966;55(11):4.
Hamill R, Olson F, Petersen R. Some interfacial properties of a nonaqueous emulsion. J Pharm Sci. 1965;54(4):4.
Suitthimeathegorn O, Jaitely V, Florence AT. Novel anhydrous emulsions: formulation as controlled release vehicles. Int J Pharm. 2005;298(2):367–71.
Atanase LI, Riess G. Block copolymer stabilized nonaqueous biocompatible sub-micron emulsions for topical applications. Int J Pharm. 2013;448(2):339–45.
Crespy D, Landfester K. Making dry fertile: a practical tour of non-aqueous emulsions and miniemulsions, their preparation and some applications. Soft Matter. 2011;7:11.
Friberg E, Podzimek M. A non-aqueous microemulsion. Colloid Polym Sci. 1984;262(3):252–3.
Harrar A, Zech O, Klaus A, Bauduin P, Kunz W. Influence of surfactant amphiphilicity on the phase behavior of IL-based microemulsions. J Colloid Interface Sci. 2011;362(2):423–9.
Charoenputtakun P, Li SK, Ngawhirunpat T. Iontophoretic delivery of lipophilic and hydrophilic drugs from lipid nanoparticles across human skin. Int J Pharm. 2015;495(1):318–28.
Magnusson BM, Cross SE, Winckle G, Roberts MS. Percutaneous absorption of steroids: determination of in vitro permeability and tissue reservoir characteristics in human skin layers. Skin Pharmacol Physiol. 2006;19(6):336–42.
Toropainen E, Ranta VP, Talvitie A, Suhonen P, Urtti A. Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest Ophthalmol Vis Sci. 2001;42(12):2942–8.
Mukai K, Tokunaga A, Itoh S, Kanesaki Y, Ohara K, Nagaoka S, et al. Structure-activity relationship of the free-radical-scavenging reaction by vitamin E (alpha-, beta-, gamma-, delta-Tocopherols) and ubiquinol-10: pH dependence of the reaction rates. J Phys Chem B. 2007;111(3):652–62.
Teichmann A, Jacobi U, Ossadnik M, Richter H, Koch S, Sterry W, et al. Differential stripping: determination of the amount of topically applied substances penetrated into the hair follicles. J Invest Dermatol. 2005;125(2):264–9.
Spernath A, Yaghmur A, Aserin A, Hoffman RE, Garti N. Food-grade microemulsions based on nonionic emulsifiers: media to enhance lycopene solubilization. J Agric Food Chem. 2002;50(23):6917–22.
Vertzoni M, Kartezini T, Reppas C, Archontaki H, Valsami G. Solubilization and quantification of lycopene in aqueous media in the form of cyclodextrin binary systems. Int J Pharm. 2006;309(1–2):115–22.
Poljsak B, Dahmane R. Free radicals and extrinsic skin aging. Dermatol Res Pract. 2012;2012:135206.
van Breemen RB, Pajkovic N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008;269(2):339–51.
Ascenso A, Pinho S, Eleuterio C, Praca FG, Bentley MV, Oliveira H, et al. Lycopene from tomatoes: vesicular nanocarrier formulations for dermal delivery. J Agric Food Chem. 2013;61(30):7284–93.
Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(1):42–50.
Kantarci G, Ozguney I, Karasulu HY, Arzik S, Guneri T. Comparison of different water/oil microemulsions containing diclofenac sodium: preparation, characterization, release rate, and skin irritation studies. AAPS PharmSciTech. 2007;8(4), E91.
Lopes LB, Murphy N, Nornoo A. Enhancement of transdermal delivery of progesterone using medium-chain mono and diglycerides as skin penetration enhancers. Pharm Dev Technol. 2009;14(5):524–9.
Hosmer J, Reed R, Bentley MV, Nornoo A, Lopes LB. Microemulsions containing medium-chain glycerides as transdermal delivery systems for hydrophilic and hydrophobic drugs. AAPS PharmSciTech. 2009;10(2):589–96.
Nornoo AO, Chow DS. Cremophor-free intravenous microemulsions for paclitaxel II. Stability, in vitro release and pharmacokinetics. Int J Pharm. 2008;349(1–2):117–23.
Jordan M, Nayel A, Brownlow B, Elbayoumi T. Development and evaluation of tocopherol-rich argan oil-based nanoemulsions as vehicles possessing anticancer activity. J Biomed Nanotechnol. 2012;8(6):944–56.
Pierre MB, Ricci Jr E, Tedesco AC, Bentley MV. Oleic acid as optimizer of the skin delivery of 5-aminolevulinic acid in photodynamic therapy. Pharm Res. 2006;23(2):360–6.
Patel N, Schmid U, Lawrence MJ. Phospholipid-based microemulsions suitable for use in foods. J Agric Food Chem. 2006;54(20):7817–24.
Hosmer JM, Steiner AA, Lopes LB. Lamellar liquid crystalline phases for cutaneous delivery of Paclitaxel: impact of the monoglyceride. Pharm Res. 2013;30(3):694–706.
Hosmer JM, Shin SH, Nornoo A, Zheng H, Lopes LB. Influence of internal structure and composition of liquid crystalline phases on topical delivery of paclitaxel. J Pharm Sci. 2011;100(4):1444–55.
Clogston J, Rathman J, Tomasko D, Walker H, Caffrey M. Phase behavior of a monoacylglycerol: (myverol 18-99K)/water system. Chem Phys Lipids. 2000;107(2):191–220.
Shah JC, Sadhale Y, Chilukuri DM. Cubic phase gels as drug delivery systems. Adv Drug Deliv Rev. 2001;47(2–3):229–50.
Wang Z, Diao Z, Liu F, Li G, Zhang G. Microstructure and rheological properties of liquid crystallines formed in Brij 97/water/IPM system. J Colloid Interface Sci. 2006;297(2):813–8.
Pepe D, Phelps J, Lewis K, Dujack J, Scarlett K, Jahan S, et al. Decylglucoside-based microemulsions for cutaneous localization of lycopene and ascorbic acid. Int J Pharm. 2012;434(1–2):420–8.
Garti N, Yaghmur A, Leser ME, Clement V, Watzke HJ. Improved oil solubilization in oil/water food grade microemulsions in the presence of polyols and ethanol. J Agric Food Chem. 2001;49(5):2552–62.
Kogan A, Aserin A, Garti N. Improved solubilization of carbamazepine and structural transitions in nonionic microemulsions upon aqueous phase dilution. J Colloid Interface Sci. 2007;315(2):637–47.
Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123–126:369–85.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45(1):89–121.
Heuschkel S, Goebel A, Neubert RH. Microemulsions--modern colloidal carrier for dermal and transdermal drug delivery. J Pharm Sci. 2008;97(2):603–31.
Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev. 2002;54 Suppl 1:S77–98.
Graf A, Ablinger E, Peters S, Zimmer A, Hook S, Rades T. Microemulsions containing lecithin and sugar-based surfactants: nanoparticle templates for delivery of proteins and peptides. Int J Pharm. 2008;350(1–2):351–60.
Krauel K, Graf A, Hook SM, Davies NM, Rades T. Preparation of poly (alkylcyanoacrylate) nanoparticles by polymerization of water-free microemulsions. J Microencapsul. 2006;23(5):499–512.
Mahdi ES, Noor AM, Sakeena MH, Abdullah GZ, Abdulkarim MF, Sattar MA. Formulation and in vitro release evaluation of newly synthesized palm kernel oil esters-based nanoemulsion delivery system for 30% ethanolic dried extract derived from local Phyllanthus urinaria for skin antiaging. Int J Nanomedicine. 2011;6:2499–512.
Lopes LB, VanDeWall H, Li HT, Venugopal V, Li HK, Naydin S, et al. Topical delivery of lycopene using microemulsions: enhanced skin penetration and tissue antioxidant activity. J Pharm Sci. 2010;99(3):1346–57.
Herwadkar A, Sachdeva V, Taylor LF, Silver H, Banga AK. Low frequency sonophoresis mediated transdermal and intradermal delivery of ketoprofen. Int J Pharm. 2012;423(2):289–96.
Mohammed D, Hirata K, Hadgraft J, Lane ME. Influence of skin penetration enhancers on skin barrier function and skin protease activity. Eur J Pharm Sci. 2014;51:118–22.
Alber C, Buraczewska-Norin I, Kocherbitov V, Saleem S, Loden M, Engblom J. Effects of water activity and low molecular weight humectants on skin permeability and hydration dynamics - a double-blind, randomized and controlled study. Int J Cosmet Sci. 2014;36(5):412–8.
Rozman B, Zvonar A, Falson F, Gasperlin M. Temperature-sensitive microemulsion gel: an effective topical delivery system for simultaneous delivery of vitamins C and E. AAPS PharmSciTech. 2009;10(1):54–61.
Rangarajan M, Zatz JL. Effect of formulation on the topical delivery of alpha-tocopherol. J Cosmet Sci. 2003;54(2):161–74.
Kandárová H, Hayden P, Klausner M, Kubilus J, Sheasgreen J. An in vitro skin irritation test (SIT) using the EpiDerm Reconstructed Human Epidermal (RHE) model. J Visual Exp. 2009;29. http://www.jove.com/index/Details.stp?ID=1366. doi: 10.3791/1366.
Brohem CA, Cardeal LB, Tiago M, Soengas MS, Barros SB, Maria-Engler SS. Artificial skin in perspective: concepts and applications. Pigment Cell Melanoma Res. 2011;24(1):35–50.
Rozman B, Gosenca M, Falson F, Gasperlin M. The influence of microemulsion structure on their skin irritation and phototoxicity potential. Int J Pharm. 2016;499(1–2):228–35.
Hathout RM, Woodman TJ, Mansour S, Mortada ND, Geneidi AS, Guy RH. Microemulsion formulations for the transdermal delivery of testosterone. Eur J Pharm Sci. 2010;40(3):188–96.
Podlogar F, Gasperlin M, Tomsic M, Jamnik A, Rogac MB. Structural characterisation of water-Tween 40/Imwitor 308-isopropyl myristate microemulsions using different experimental methods. Int J Pharm. 2004;276(1–2):115–28.
Todosijevic MN, Savic MM, Batinic BB, Markovic BD, Gasperlin M, Randelovic DV, et al. Biocompatible microemulsions of a model NSAID for skin delivery: a decisive role of surfactants in skin penetration/irritation profiles and pharmacokinetic performance. Int J Pharm. 2015;496(2):931–41.
Kogan A, Shalev DE, Raviv U, Aserin A, Garti N. Formation and characterization of ordered bicontinuous microemulsions. J Phys Chem B. 2009;113(31):10669–78.
Malheiro AR, Varanda LC, Perez J, Villullas HM. The aerosol OT+n-butanol+n-heptane+water system: phase behavior, structure characterization, and application to Pt70Fe30 nanoparticle synthesis. Langmuir. 2007;23(22):11015–20.
Gee CM, Nicolazzo JA, Watkinson AC, Finnin BC. Assessment of the lateral diffusion and penetration of topically applied drugs in humans using a novel concentric tape stripping design. Pharm Res. 2012;29(8):2035–46.
Lopes LB, Reed R. A simple and rapid method to assess lycopene in multiple layers of skin samples. Biomed Chromatogr. 2010;24(2):154–9.
Mahrhauser D, Nagelreiter C, Baierl A, Skipiol J, Valenta C. Influence of a multiple emulsion, liposomes and a microemulsion gel on sebum, skin hydration and TEWL. Int J Cosmet Sci. 2015;37(2):181–6.
Herkenne C, Naik A, Kalia YN, Hadgraft J, Guy RH. Effect of propylene glycol on ibuprofen absorption into human skin in vivo. J Pharm Sci. 2008;97(1):185–97.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56(5):603–18.
Khandavilli S, Panchagnula R. Nanoemulsions as versatile formulations for paclitaxel delivery: peroral and dermal delivery studies in rats. J Invest Dermatol. 2007;127(1):154–62.
Bernabeu E, Gonzalez L, Cagel M, Gergic EP, Moretton MA, Chiappetta DA. Novel Soluplus((R))-TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf B: Biointerfaces. 2016;140:403–11.
Wartewig S, Neubert RH. Properties of ceramides and their impact on the stratum corneum structure: a review. Part 1: ceramides. Skin Pharmacol Physiol. 2007;20(5):220–9.
Wagner H, Kostka KH, Adelhardt W, Schaefer UF. Effects of various vehicles on the penetration of flufenamic acid into human skin. Eur J Pharm Biopharm. 2004;58(1):121–9.
Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Med. 2006;72(4):311–6.
Avdeef A, Bendels S, Tsinman O, Tsinman K, Kansy M. Solubility-excipient classification gradient maps. Pharm Res. 2007;24(3):530–45.
El Maghraby GM. Occlusive and non-occlusive application of microemulsion for transdermal delivery of progesterone: mechanistic studies. Sci Pharm. 2012;80(3):765–78.
Dubbs MD, Gupta RB, 590. Solubility of vitamin E (a-Tocopherol) and vitamin K3 (Menadione) in ethanol-water mixture. J Chem Eng Data. 1998;43(590):2.
Djekic L, Martinovic M, Stepanovic-Petrovic R, Tomic M, Micov A, Primorac M. Design of block copolymer costabilized nonionic microemulsions and their in vitro and in vivo assessment as carriers for sustained regional delivery of ibuprofen via topical administration. J Pharm Sci. 2015;104(8):2501–12.
Cavalcanti AL, Reis MY, Silva GC, Ramalho IM, Guimaraes GP, Silva JA, et al. Microemulsion for topical application of pentoxifylline: in vitro release and in vivo evaluation. Int J Pharm. 2016;506(1–2):351–60.
Erdal MS, Ozhan G, Mat MC, Ozsoy Y, Gungor S. Colloidal nanocarriers for the enhanced cutaneous delivery of naftifine: characterization studies and in vitro and in vivo evaluations. Int J Nanomedicine. 2016;11:1027–37.
Mishra R, Prabhavalkar KS, Bhatt LK. Preparation, optimization, and evaluation of Zaltoprofen-loaded microemulsion and microemulsion-based gel for transdermal delivery. J Liposome Res. 2016:1–10.
Sharma G, Dhankar G, Thakur K, Raza K, Katare OP. Benzyl benzoate-loaded microemulsion for topical applications: enhanced dermatokinetic profile and better delivery promises. AAPS PharmSciTech; 2015.
Nasr M, Abdel-Hamid S. Optimizing the dermal accumulation of a tazarotene microemulsion using skin deposition modeling. Drug Dev Ind Pharm. 2016;42(4):636–43.
Zhao L, Wang Y, Zhai Y, Wang Z, Liu J, Zhai G. Ropivacaine loaded microemulsion and microemulsion-based gel for transdermal delivery: preparation, optimization, and evaluation. Int J Pharm. 2014;477(1–2):47–56.
Acknowledgments
This study was supported by São Paulo Research Foundation (FAPESP grant#2013/16617-7) and PhRMA Foundation (Research Starter Grant in Pharmaceutics). Fellowships from CAPES (V. Carvalho) and FAPESP (D. de Lemos and A. Migotto) are greatly appreciated. We would like to thank Dr. A. Steiner for critical comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vanessa F. Carvalho and Debora P. de Lemos contributed equally to this work.
Rights and permissions
About this article
Cite this article
Carvalho, V.F., de Lemos, D.P., Vieira, C.S. et al. Potential of Non-aqueous Microemulsions to Improve the Delivery of Lipophilic Drugs to the Skin. AAPS PharmSciTech 18, 1739–1749 (2017). https://doi.org/10.1208/s12249-016-0643-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0643-7